2023-10-11 シンガポール国立大学(NUS)
◆この新しい触媒は、炭素排出量を10倍低減し、微細な化学物質と医薬品の製造における魅力的な選択肢となります。将来的には、新たなライブラリの開発を通じて環境に優しい製造プロセスを実現し、新しい化学物質や医薬品の時代を切り拓く計画です。
<関連情報>
- https://news.nus.edu.sg/novel-catalyst-for-green-production-of-fine-chemicals-and-pharmaceuticals/
- https://www.nature.com/articles/s41586-023-06529-z
クロスカップリングのためのジェミナル原子触媒作用 Geminal-atom catalysis for cross-coupling
Xiao Hai,Yang Zheng,Qi Yu,Na Guo,Shibo Xi,Xiaoxu Zhao,Sharon Mitchell,Xiaohua Luo,Victor Tulus,Mu Wang,Xiaoyu Sheng,Longbin Ren,Xiangdong Long,Jing Li,Peng He,Huihui Lin,Yige Cui,Xinnan Peng,Jiwei Shi,Jie Wu,Chun Zhang,Ruqiang Zou,Gonzalo Guillén-Gosálbez,Javier Pérez-Ramírez,Ming Joo Koh,Ye Zhu,Jun Li & Jiong Lu
Nature Published:20 September 2023
DOI:https://doi.org/10.1038/s41586-023-06529-z
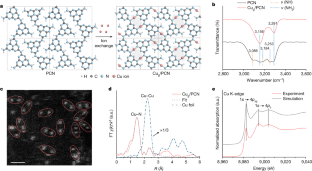
Abstract
Single-atom catalysts (SACs) have well-defined active sites, making them of potential interest for organic synthesis1,2,3,4. However, the architecture of these mononuclear metal species stabilized on solid supports may not be optimal for catalysing complex molecular transformations owing to restricted spatial environment and electronic quantum states5,6. Here we report a class of heterogeneous geminal-atom catalysts (GACs), which pair single-atom sites in specific coordination and spatial proximity. Regularly separated nitrogen anchoring groups with delocalized π-bonding nature in a polymeric carbon nitride (PCN) host7 permit the coordination of Cu geminal sites with a ground-state separation of about 4 Å at high metal density8. The adaptable coordination of individual Cu sites in GACs enables a cooperative bridge-coupling pathway through dynamic Cu–Cu bonding for diverse C–X (X = C, N, O, S) cross-couplings with a low activation barrier. In situ characterization and quantum-theoretical studies show that such a dynamic process for cross-coupling is triggered by the adsorption of two different reactants at geminal metal sites, rendering homo-coupling unfeasible. These intrinsic advantages of GACs enable the assembly of heterocycles with several coordination sites, sterically congested scaffolds and pharmaceuticals with highly specific and stable activity. Scale-up experiments and translation to continuous flow suggest broad applicability for the manufacturing of fine chemicals.