2025-05-22 韓国基礎科学研究院 (IBS)
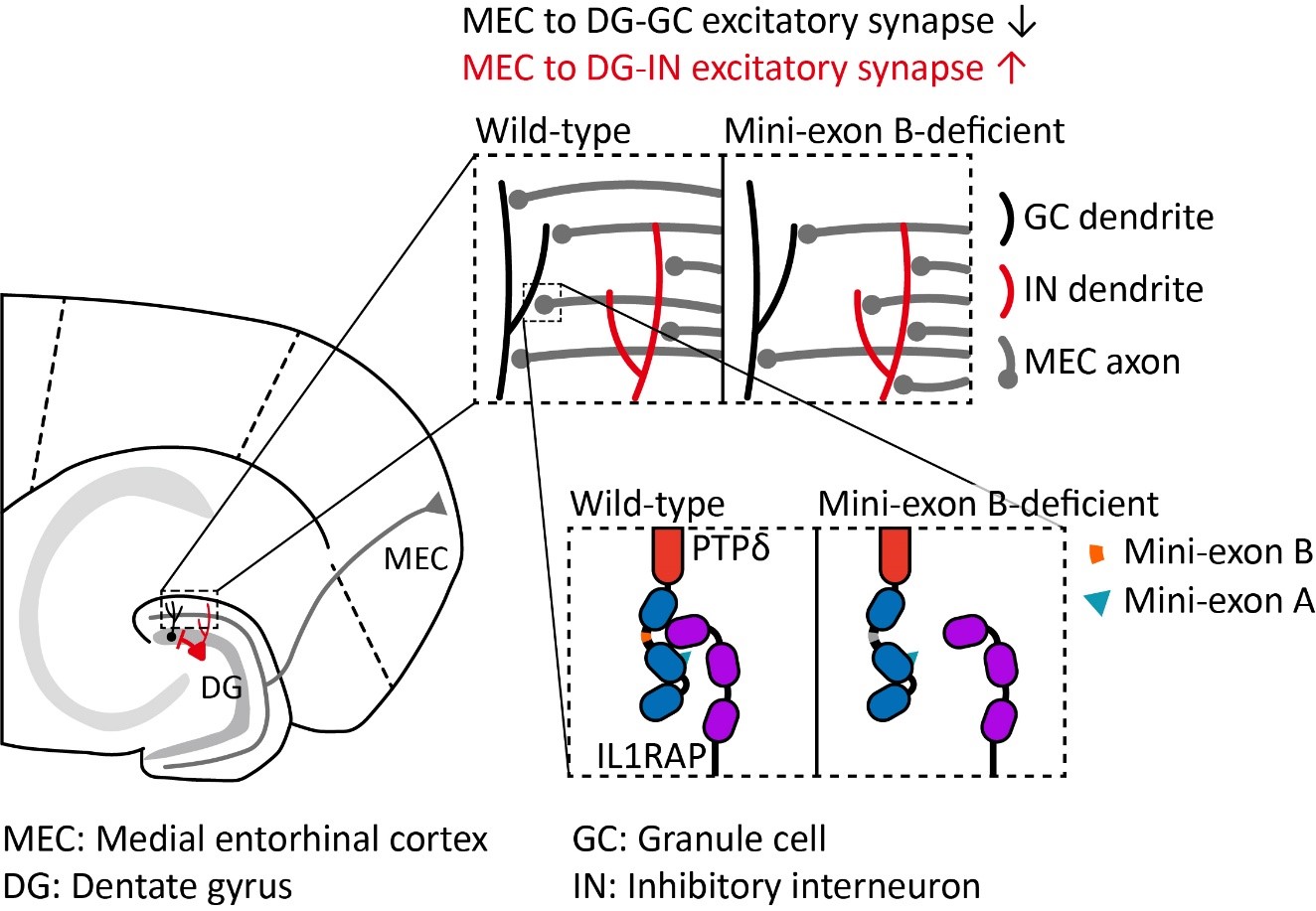 Figure 1. Excitation-Inhibition Balance Regulated by Mini-Exon B of PTPδ
Figure 1. Excitation-Inhibition Balance Regulated by Mini-Exon B of PTPδ
(Deletion of mini-exon B in the synaptic adhesion molecule PTPδ leads to altered neural connectivity from the entorhinal cortex to the dentate gyrus (left panel). In mice lacking mini-exon B, excitatory input onto granule cells is reduced, while excitatory input onto inhibitory interneurons is increased (top right panel). At the molecular level, this is likely due to a reduced trans-synaptic interaction between PTPδ and IL1RAP at excitatory synapses on granule cell dendrites (bottom right panel).
<関連情報>
- https://www.ibs.re.kr/cop/bbs/BBSMSTR_000000000738/selectBoardArticle.do?nttId=25845
- https://www.nature.com/articles/s41467-025-59685-3
PTPδの交互スプライシングミニエクソンBは、細胞型特異的な経シナプスPTPδ-IL1RAP相互作用を通して興奮性シナプスを制御する Alternatively spliced mini-exon B in PTPδ regulates excitatory synapses through cell-type-specific trans-synaptic PTPδ-IL1RAP interaction
Seoyeong Kim,Jae Jin Shin,Muwon Kang,Yeji Yang,Yi Sul Cho,Hyojung Paik,Jimin Kim,Yunho Yi,Suho Lee,Hei Yeun Koo,Jinwoong Bok,Yong Chul Bae,Jin Young Kim & Eunjoon Kim
Nature Communications Published:13 May 2025
DOI:https://doi.org/10.1038/s41467-025-59685-3
Abstract
PTPδ, encoded by PTPRD, is implicated in various neurological, psychiatric, and neurodevelopmental disorders, but the underlying mechanisms remain unclear. PTPδ trans-synaptically interacts with multiple postsynaptic adhesion molecules, which involves its extracellular alternatively spliced mini-exons, meA and meB. While PTPδ-meA functions have been studied in vivo, PTPδ-meB has not been studied. Here, we report that, unlike homozygous PTPδ-meA-mutant mice, homozygous PTPδ-meB-mutant (Ptprd-meB–/–) mice show markedly reduced early postnatal survival. Heterozygous Ptprd-meB+/– male mice show behavioral abnormalities and decreased excitatory synaptic density and transmission in dentate gyrus granule cells (DG-GCs). Proteomic analyses identify decreased postsynaptic density levels of IL1RAP, a known trans-synaptic partner of meB-containing PTPδ. Accordingly, IL1RAP-mutant mice show decreased excitatory synaptic transmission in DG-GCs. Ptprd-meB+/– DG interneurons with minimal IL1RAP expression show increased excitatory synaptic density and transmission. Therefore, PTPδ-meB is important for survival, synaptic, and behavioral phenotypes and regulates excitatory synapses in cell-type-specific and IL1RAP-dependent manners.


