2025-10-08 マサチューセッツ工科大学 (MIT)
<関連情報>
- https://news.mit.edu/2025/engineered-natural-killer-cells-could-help-fight-cancer-1008
- https://www.nature.com/articles/s41467-025-63863-8
選択的HLAノックダウンとPD-L1発現は、同種CAR-NK細胞の拒絶を防ぎ、異種移植マウスにおける安全性と抗腫瘍反応を強化する Selective HLA knockdown and PD-L1 expression prevent allogeneic CAR-NK cell rejection and enhance safety and anti-tumor responses in xenograft mice
Fuguo Liu,Mubin Tarannum,Yingjie Zhao,Yiming J. Zhang,James Dongjoo Ham,Kewen Lei,Yuhao Qiang,Xingyu Deng,Maily Nguyen,Khanhlinh Dinh,Shaobo Yang,Alaa Kassim Ali,Toni K. Choueiri,Jerome Ritz,Rizwan Romee & Jianzhu Chen
Nature Communications Published:08 October 2025
DOI:https://doi.org/10.1038/s41467-025-63863-8
Abstract
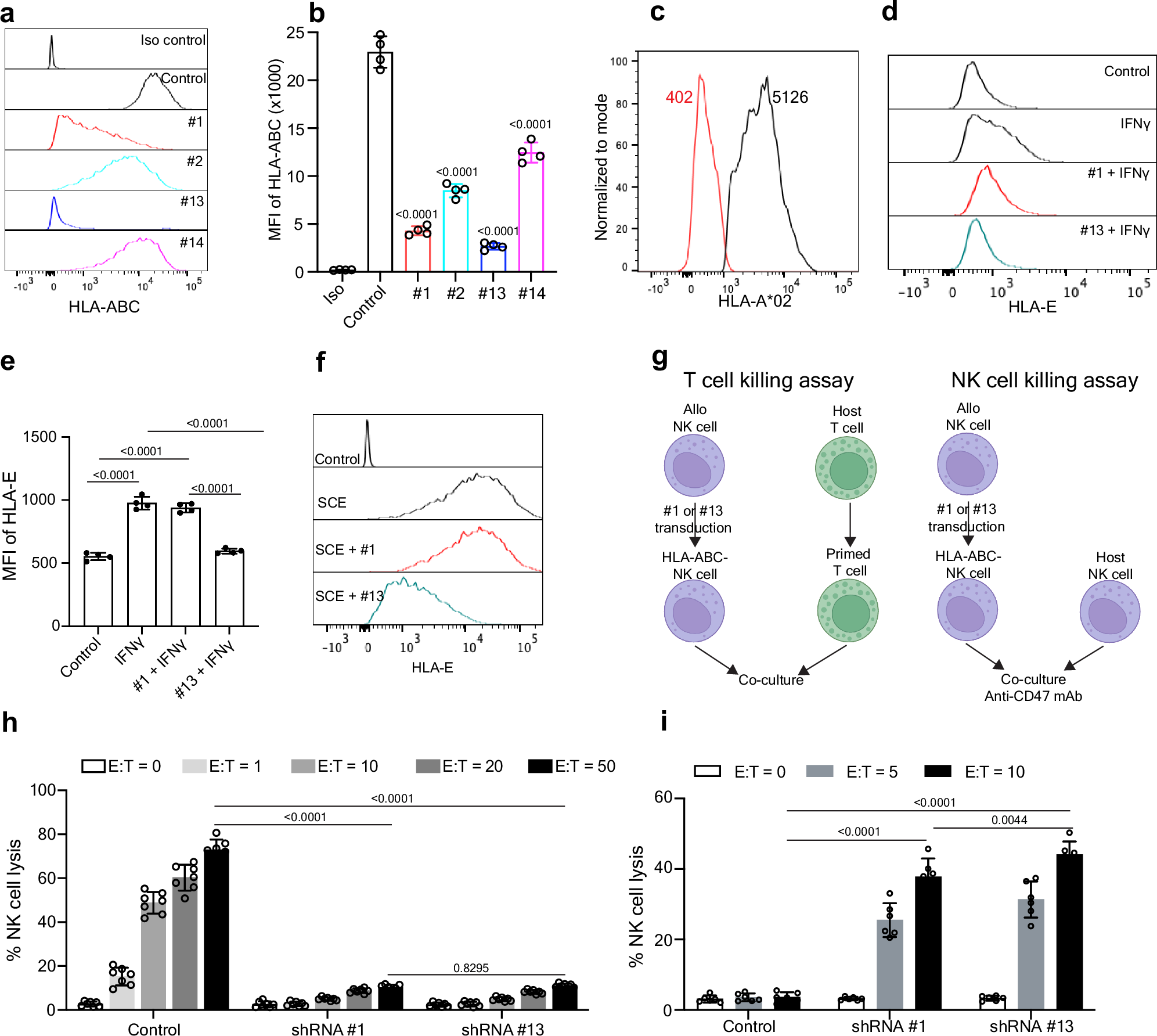
Allogeneic cellular immunotherapy exhibits promising efficacy for cancer treatment, but donor cell rejection remains a major barrier. Here, we systematically evaluate human leukocyte antigens (HLA) and immune checkpoints PD-L1, HLA-E, and CD47 in the rejection of allogeneic NK cells and identify CD8+ T cells as the dominant cell type mediating allorejection. We demonstrate that a single gene construct that combines an shRNA that selectively interferes with HLA class I but not HLA-E expression, a chimeric antigen receptor (CAR), and PD-L1 or single-chain HLA-E (SCE) enables the one-step construction of allogeneic CAR-NK cells that evade host-mediated rejection both in vitro and in a xenograft mouse model. Furthermore, CAR-NK cells overexpressing PD-L1 or SCE effectively kill tumor cells through the upregulation of cytotoxic genes and reduced exhaustion and exhibit a favorable safety profile due to the decreased production of inflammatory cytokines involved in cytokine release syndrome. Thus, our approach represents a promising strategy in enabling “off-the-shelf” allogeneic cellular immunotherapies.