2025-12-18 ミュンヘン大学(LMU)
<関連情報>
- https://www.lmu.de/en/newsroom/news-overview/news/human-model-of-blood-brain-barrier-a3af2e3b.html
- https://www.nature.com/articles/s41593-025-02123-w
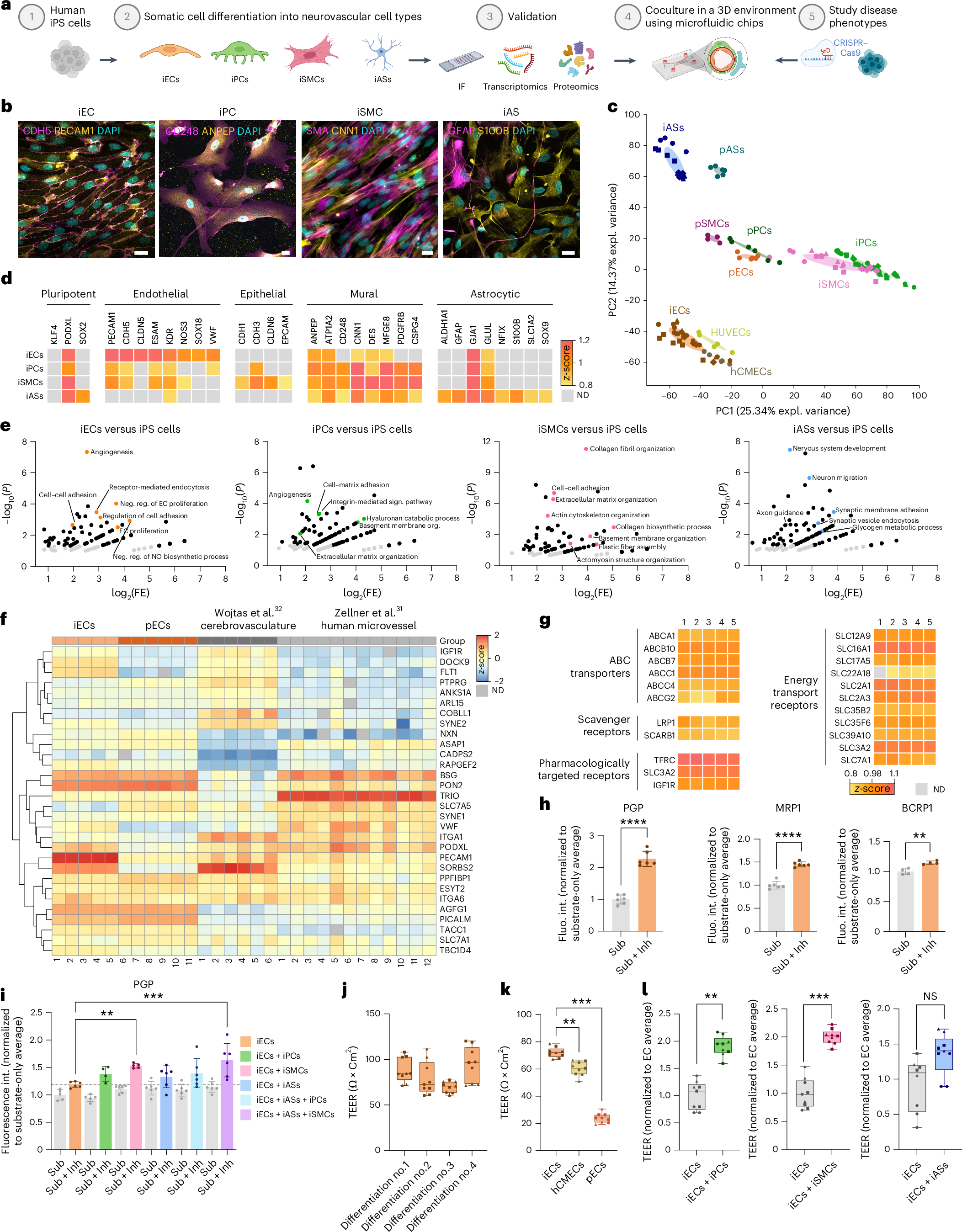
神経血管疾患のメカニズムと治療介入を研究するための、ヒト血液脳関門の完全iPS細胞由来3Dモデル A fully iPS-cell-derived 3D model of the human blood–brain barrier for exploring neurovascular disease mechanisms and therapeutic interventions
Judit González-Gallego,Katalin Todorov-Völgyi,Stephan A. Müller,Sophie Antesberger,Mihail Ivilinov Todorov,Rainer Malik,Rita Grimalt-Mirada,Carolina Cardoso Gonçalves,Martina Schifferer,Georg Kislinger,Isabel Weisheit,Barbara Lindner,Dennis Crusius,Joseph Kroeger,Mila Borri,Ali Erturk,Mark Nelson,Thomas Misgeld,Stefan F. Lichtenthaler,Martin Dichgans & Dominik Paquet
Nature Neuroscience Published:15 December 2025
DOI:https://doi.org/10.1038/s41593-025-02123-w
Abstract
Blood–brain barrier (BBB) integrity is critical for brain homeostasis, with malfunctions contributing to neurovascular and neurodegenerative disorders. Mechanistic studies on BBB function have been mostly conducted in rodent and in vitro models, which recapitulate some disease features, but have limited translatability to humans and pose challenges for drug discovery. Here we report on a fully human induced pluripotent stem (iPS)-cell-derived, microfluidic three-dimensional (3D) BBB model consisting of endothelial cells (ECs), mural cells and astrocytes. Our model expresses typical fate markers, forms a barrier in vessel-like tubes and enables perfusion, including with human blood. Deletion of FOXF2 in ECs, a major risk gene for cerebral small vessel disease, induced key features of BBB dysfunction, including compromised cell junction integrity and enhanced caveolae formation. Proteomic analysis revealed dysregulated endocytosis and cell junction pathways. Disease features phenocopied those seen in mice with EC-specific Foxf2 deficiency. Moreover, lipid-nanoparticle-based treatment with Foxf2 mRNA rescued BBB deficits, demonstrating the potential for drug development.


