2026-02-09 中国科学院(CAS)
<関連情報>
- https://english.cas.cn/newsroom/research-news/202602/t20260213_1150965.shtml
- https://www.nature.com/articles/s41421-025-00864-3
L1td1による内因性レトロウイルスの転写後制御は多能性幹細胞における全能性獲得を抑制する
Post-transcriptional control of endogenous retroviruses by L1td1 suppresses totipotency acquisition in pluripotent stem cells
Yi Wu,Yang Liu,Yile Huang,Zhihong Hao,Wenxin Li,Yukun Li,Maolei Zhang,Linpeng Li,Dajiang Qin,Keshi Chen & Xingguo Liu
Nature Discovery Published:20 January 2026
DOI:https://doi.org/10.1038/s41421-025-00864-3
Dear Editor,
Early mammalian embryogenesis begins with a fertilized egg and zygotic genome activation (ZGA), the switch from maternal to zygotic control. In mouse two-cell (2C) embryos, ZGA is characterized by the transient activation of murine endogenous retrovirus-L (MERVL) and 2C-specific genes, such as Zscan41. As development proceeds, expression of these genes declines as cells transition from a totipotent state, capable of producing all lineages, including extra-embryonic tissues, to a pluripotent state restricted to the three germ layers. Totipotency and pluripotency are dynamic cellular states central to development and hold great promise for both fundamental research and clinical applications in regenerative medicine1. Embryonic stem cells (ESCs) derived from the inner cell mass of blastocysts exhibit pluripotency, and a rare subpopulation termed two-cell-like cells (2CLCs) spontaneously emerges, recapitulating key molecular features and developmental potentials of 2C-stage embryos2. The molecular mechanisms governing ZGA and the transitions between pluripotency and totipotency have been increasingly elucidated across multiple regulatory layers, particularly at the transcriptional, epigenetic, and metabolic levels1,3,4. Recent studies reported that spliceosomal repression reprograms both human and mouse pluripotent stem cells (PSCs) toward a totipotent state5, suggesting an unexpected layer of post-transcriptional regulation. However, the precise post-transcriptional mechanisms governing pluripotency-totipotency transitions remain unclear.
RNA-binding proteins (RBPs) mediate post-transcriptional control of mRNA stability, localization, and translation, thereby influencing stem cell fate. Long interspersed nuclear element 1 (LINE1)-type transposase domain containing 1 (L1td1) is the only domesticated protein-coding gene almost entirely derived from the LINE1 (L1) retroelement6. L1td1 is highly expressed in PSCs and has been reported to be essential for maintaining pluripotency in human cells7. Intriguingly, evolutionary analyses suggest that L1td1 originated under positive selection in primates and rodents and was later co-opted into pluripotency networks6. Recent studies further reveal that L1td1 interacts with ancestral L1 ORF1p to facilitate L1 retrotransposition in cancer cells8. Despite links to transposable elements (TEs) and pluripotency, L1td1’s role in totipotency remains unexplored.
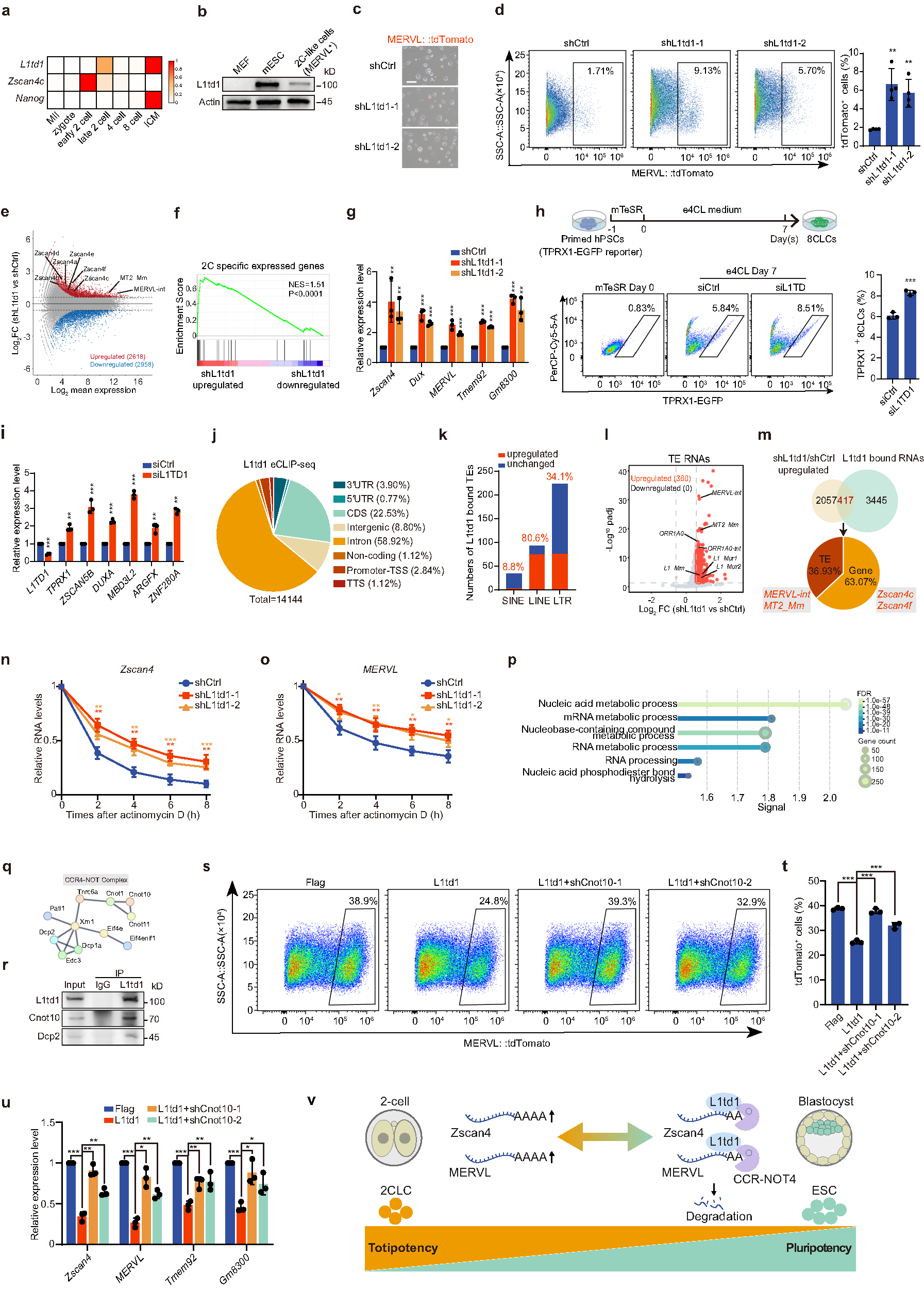
To investigate the potential role of L1td1 in embryonic development, we analyzed its expression across different developmental stages from single-embryo RNA-seq and proteomic datasets9,10. L1td1 peaks at the late 2C stage when Zscan4c declines, and rises again in the inner cell mass, a trend also reflected at the protein level (Fig. 1a; Supplementary Fig. S1a), suggesting that L1td1 may play roles in both exit from totipotency and maintenance of pluripotency. We also assessed L1td1 protein expression in mouse embryonic fibroblasts (MEFs), ESCs, and 2CLCs induced by Dux. The results showed that the expression of L1td1 was high in ESCs but low in MEFs and 2CLCs (Fig. 1b). We then knocked down L1td1 in ESCs harboring the 2C reporter MERVL::tdTomato and observed an increase in the proportion of MERVL+ cells (Fig. 1c; Supplementary Fig. S1b), which was confirmed by flow cytometry analysis (Fig. 1d). Transcriptomic analysis revealed that L1td1 knockdown upregulated the expression of totipotency genes, including Zscan4 family and ZGA-associated endogenous retroelements, MERVL-int (internal) and MT2_Mm (MERVL long terminal repeat (LTR)) (Fig. 1e; Supplementary Fig. S1c). Gene set enrichment analysis (GSEA) indicated an upregulation of 2C-specific genes following L1td1 knockdown (Fig. 1f). Finally, qPCR analysis confirmed the increased expression of totipotency genes (Fig. 1g). In vitro embryo culture showed that L1td1 knockdown increased the proportion of embryos arrested at the 2C and 4C stages, indicating that L1td1 contributes to the transition from totipotency to pluripotency (Supplementary Fig. S1d).