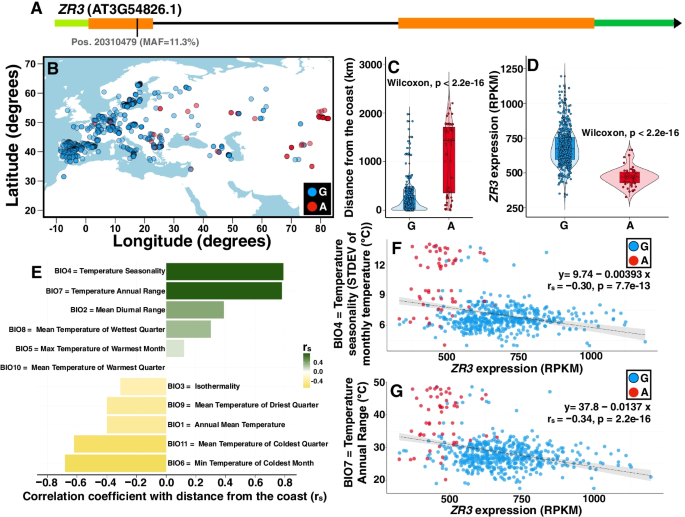
2022-06-13 ペンシルベニア州立大学(PennState)
 A Penn State-led research team found that robust, customizable biotechnology platform-based techniques were more quickly adopted to manufacture COVID-19 vaccines than traditional vaccine development and manufacturing approaches. Credit: solarseven/iStock. All Rights Reserved.
A Penn State-led research team found that robust, customizable biotechnology platform-based techniques were more quickly adopted to manufacture COVID-19 vaccines than traditional vaccine development and manufacturing approaches. Credit: solarseven/iStock. All Rights Reserved.
ペンシルベニア州立大学が主導するチームがこの移行を検証した結果、このようなスマートな製造技術は将来的に他のウイルスにも適用でき、常に進化する病原体に対応したワクチン開発が可能になる可能性があると、プロジェクトリーダーのSoundar Kumara(Allen E. Pearce and Allen M. Pearce Professor of Industrial Engineering at Penn State)は結論付けている。
この研究成果は、米国機械学会誌「Journal of Computing and Information Science in Engineering」のオンライン版に掲載され、同誌の8月発行の印刷版に掲載される予定です。
<関連情報>
- https://www.psu.edu/news/research/story/biotechnology-platforms-enable-fast-customizable-vaccine-production/
- https://asmedigitalcollection.asme.org/computingengineering/article-abstract/22/4/040903/1129387/Smart-Vaccine-Manufacturing-Using-Novel
新規バイオテクノロジープラットフォームを用いたスマートなワクチン製造。COVID-19での研究 Smart Vaccine Manufacturing Using Novel Biotechnology Platforms: A Study During COVID-19
Vishnu Kumar,Vijay Srinivasan,Soundar Kumara
Journal of Computing and Information Science in Engineering Published: February 16, 2022
DOI:https://doi.org/10.1115/1.4053273
Abstract
Healthcare experts have come to a consensus that effective and safe vaccines are necessary to control the rapid spread of the ongoing COVID-19 pandemic across the globe. Since the traditional vaccine development and manufacturing approaches were unable to meet the rapidly growing COVID-19 vaccine demand, biopharmaceutical firms had to devise novel and smart techniques to boost the development, production, and distribution of COVID-19 vaccines in a large scale with lightning speed. This triggered their transition to smart vaccine manufacturing approaches using novel viral vector and nucleic acid biotechnology platforms. This paper tries to explore this rationality of the biopharmaceutical industry by comparing the traditional and the novel biotechnology platform-based vaccine manufacturing techniques and reviewing the COVID-19 vaccine manufacturing scenarios. To highlight the “smart” characteristics of the novel platform-based COVID-19 vaccine products and to make an effective comparison with the traditional products, a well-established product classification framework is used as a reference. Finally, the study concludes by presenting the future possibility of incorporating smart manufacturing paradigms with the novel platform-based manufacturing process. It is hoped that this study would serve as an asset for the biopharmaceutical firms to appropriately streamline their strategies, resources, and goals to meet the global vaccine requirements.