2023-07-07 カリフォルニア大学バークレー校(UCB)
◆この発見は、睡眠を利用した糖尿病治療の可能性を示唆しており、深い睡眠の脳波は血糖コントロールの予測に有用なマーカーとなることも明らかになりました。これにより、新たな治療法や非侵襲的な手法の開発が期待され、糖尿病患者の血糖管理の向上につながる可能性があります。
<関連情報>
- https://news.berkeley.edu/2023/07/07/new-research-finds-deep-sleep-brain-waves-predict-blood-sugar-control/
- https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(23)00219-7
- https://www.nature.com/articles/s41586-021-03811-w
- https://www.sciencedirect.com/science/article/pii/S096098221100042X
ヒトの睡眠脳波が末梢のグルコースホメオスタシスをマッピングする Coordinated human sleeping brainwaves map peripheral body glucose homeostasis
Raphael Vallat,Vyoma D. Shah,Matthew P. Walker
Cell Reports Medicine Published:July 07, 2023
DOI:https://doi.org/10.1016/j.xcrm.2023.101100

Highlights
•Coupled NREM sleep brainwaves predict superior next-day glucose control in two cohorts
•This sleep-glucose association is partially mediated by autonomic activity
•Sleep may regulate glycemic status selectively via insulin sensitivity
•NREM brainwaves thus offer a glycemic biomarker and a potential therapeutic target
Summary
Insufficient sleep impairs glucose regulation, increasing the risk of diabetes. However, what it is about the human sleeping brain that regulates blood sugar remains unknown. In an examination of over 600 humans, we demonstrate that the coupling of non-rapid eye movement (NREM) sleep spindles and slow oscillations the night before is associated with improved next-day peripheral glucose control. We further show that this sleep-associated glucose pathway may influence glycemic status through altered insulin sensitivity, rather than through altered pancreatic beta cell function. Moreover, we replicate these associations in an independent dataset of over 1,900 adults. Of therapeutic significance, the coupling between slow oscillations and spindles was the most significant sleep predictor of next-day fasting glucose, even more so than traditional sleep markers, relevant to the possibility of an electroencephalogram (EEG) index of hyperglycemia. Taken together, these findings describe a sleeping-brain-body framework of optimal human glucose homeostasis, offering a potential prognostic sleep signature of glycemic control.
海馬鋭波リップルの代謝機能 A metabolic function of the hippocampal sharp wave-ripple
David Tingley,Kathryn McClain,Ekin Kaya,Jordan Carpenter & György Buzsáki
Nature Published:11 August 2021
DOIhttps://doi.org/10.1038/s41586-021-03811-w
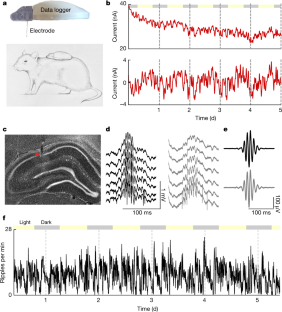
Abstract
The hippocampus has previously been implicated in both cognitive and endocrine functions1,2,3,4,5,6,7,8,9,10,11,12,13,14,15. We simultaneously measured electrophysiological activity from the hippocampus and interstitial glucose concentrations in the body of freely behaving rats to identify an activity pattern that may link these disparate functions of the hippocampus. Here we report that clusters of sharp wave-ripples recorded from the hippocampus reliably predicted a decrease in peripheral glucose concentrations within about 10 min. This correlation was not dependent on circadian, ultradian or meal-triggered fluctuations, could be mimicked with optogenetically induced ripples in the hippocampus (but not in the parietal cortex) and was attenuated to chance levels by pharmacogenetically suppressing activity of the lateral septum, which is the major conduit between the hippocampus and the hypothalamus. Our findings demonstrate that a function of the sharp wave-ripple is to modulate peripheral glucose homeostasis, and offer a mechanism for the link between sleep disruption and blood glucose dysregulation in type 2 diabetes16,17,18.
ヒトの学習における覚醒状態の悪化と睡眠の回復 Wake deterioration and sleep restoration of human learning
Bryce A. Mander, Sangeetha Santhanam , Jared M. Saletin , Matthew P. Walker
Current Biology Available online :7 March 2011
DOI:https://doi.org/10.1016/j.cub.2011.01.019

Summary
While the benefit of sleep after learning in offline consolidation is established [1], a role for sleep before learning in promoting initial memory formation remains largely uncharacterized. Existing theoretical frameworks speculate that accrued time awake, associated with ongoing experience, decreases learning capacity, while specific non-rapid-eye-movement (NREM) oscillations support restoration of learning ability 1, 2. Despite these model predictions, it remains untested whether episodic learning capacity remains stable across the day, or is progressively compromised by continued time awake. Furthermore, it is similarly unclear whether the presence, rather than the detrimental absence, of sleep restores efficient learning ability, and if so, what aspect(s) of sleep physiology support such reinstatement 3, 4. We have tested these related hypotheses, and report here a learning interaction, such that episodic encoding capacity deteriorates across a daytime waking interval, but sleep and associated NREM spindle oscillations restore efficient learning ability.