2024-12-18 ミュンヘン大学(LMU)
<関連情報>
- https://www.lmu.de/en/newsroom/news-overview/news/histone-modification-important-for-correct-blood-cell-formation.html
- https://www.pnas.org/doi/10.1073/pnas.2409656121
ヒストンメチル化酵素SETDB1は、クリプティック・エンハンサーの活性化を抑制することでマウス胎児の造血を保護する Histone methyltransferase SETDB1 safeguards mouse fetal hematopoiesis by suppressing activation of cryptic enhancers
Maryam Kazerani, Filippo Cernilogar, Alessandra Pasquarella, +3, and Gunnar Schotta
Proceedings of the National Academy of Sciences Published:December 17, 2024
DOI:https://doi.org/10.1073/pnas.2409656121

Significance
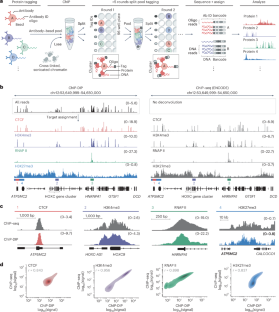
Our data show that histone methyltransferase SETDB1 is critical for preventing transcription factor (TF) activity on nonphysiological binding sites, in part overlapping with mouse endogenous retrovirus sequences. When SETDB1 is lacking, these cryptic enhancers can become overly active, leading to abnormal expression of nearby genes. This disruption coincides with compromised function of stem cells, altered differentiation of blood cell types, and increased hematopoietic stem cell proliferation. Notably, we demonstrate that SETDB1 acts as a guardian, restricting the unwarranted activity of TFs on these cryptic enhancers and maintaining the proper balance and differentiation of mouse fetal liver hematopoietic stem and progenitor cells.
Abstract
The H3K9me3-specific histone methyltransferase SETDB1 is critical for proper regulation of developmental processes, but the underlying mechanisms are only partially understood. Here, we show that deletion of Setdb1 in mouse fetal liver hematopoietic stem and progenitor cells (HSPCs) results in compromised stem cell function, enhanced myeloerythroid differentiation, and impaired lymphoid development. Notably, Setdb1-deficient HSPCs exhibit reduced quiescence and increased proliferation, accompanied by the acquisition of a lineage-biased transcriptional program. In Setdb1-deficient HSPCs, we identify genomic regions that are characterized by loss of H3K9me3 and increased chromatin accessibility, suggesting enhanced transcription factor (TF) activity. Interestingly, hematopoietic TFs like PU.1 bind these cryptic enhancers in wild-type HSPCs, despite the H3K9me3 status. Thus, our data indicate that SETDB1 restricts activation of nonphysiological TF binding sites which helps to ensure proper maintenance and differentiation of fetal liver HSPCs.