2025-07-10 マサチューセッツ工科大学(MIT)
<関連情報>
- https://news.mit.edu/2025/bionic-knee-integrated-into-tissue-can-restore-natural-movement-0710
- https://www.science.org/doi/10.1126/science.adv3223
- https://medibio.tiisys.com/129388/
組織統合型バイオニック膝が切断後の多彩な足の動きを回復させる Tissue-integrated bionic knee restores versatile legged movement after amputation
Tony Shu, Daniel Levine, Seong Ho Yeon, Ethan Chun, […] , and Hugh Herr
Science Published:10 Jul 2025
DOI:https://doi.org/10.1126/science.adv3223
Editor’s summary
Early designs for lower limb prosthetics focused on restoring basic movement. There have been advances enabling greater functionality, but these systems are still based on preprogrammed motions using limbs physically separated from the body. Shu et al. hypothesized that if connections could be formed between the soft and hard tissues and the lower leg prosthetic, then this would lead to greater stability and interfacing with the nervous system and enable more flexible and dynamic control (see the Perspective by Fisher). The authors demonstrated this concept by combining an above-knee prosthetic with an osseointegrated mount and an implanted control system to myoelectrially control the knee joint. With minimal training, the additional feedback provided to and from the knee made it easier for test subjects to avoid obstacles. —Marc S. Lavine
Structured Abstract
INTRODUCTION
Attempts to restore the body after amputation have been widespread for centuries. Typical approaches have emphasized iterative mechanical improvements, which have resulted from the difficulties and cultural aversions associated with surgical revision. Today, the most advanced, commercially available lower-extremity prostheses are still physically separated from the body and only capable of repetitive walking patterns and preprogrammed movements. By contrast, recent studies show that providing even a modest amount of neuromuscular information to the prosthesis through a minimally invasive bionic interface can unlock essential lower-extremity capabilities, such as terrain adaptation, obstacle avoidance, and biomimetic power generation. The results suggest that further anatomical integration may yield additional legged agility.
RATIONALE
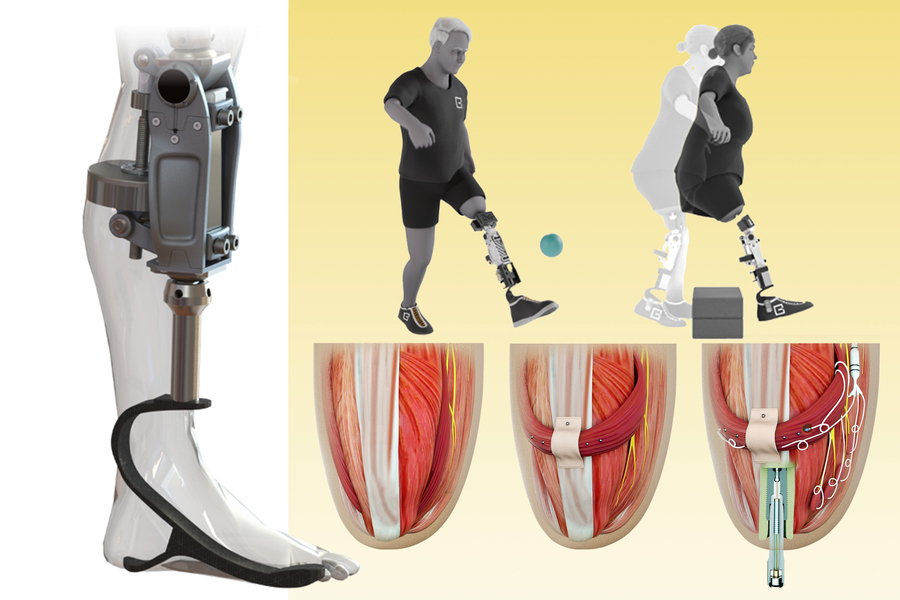
We hypothesized that engineering residual soft and hard tissues toward a seamless interface between the nervous system and prosthesis would enable versatile lower-extremity behaviors driven by user intent. We developed a vertically integrated bionic amputation platform, the osseointegrated mechanoneural prosthesis, to improve control signal detection and neuromuscular function at the above-knee amputation level. The osseointegrated mechanoneural prosthesis consists of three functional layers that seek to (i) maximize prosthetic knee agility through continuous, user-controlled flexion and extension; (ii) stabilize two-way energy and information exchange between user and prosthesis through a bone-anchored femur implant; and (iii) restore physiological neuromuscular signaling by reconstructing original muscle geometries.
RESULTS
Two human subjects with preexisting conventional transfemoral amputation were surgically enhanced with the osseointegrated mechanoneural prosthesis. We found that they demonstrated significantly improved performance relative to a cohort of subjects with conventional transfemoral amputation when controlling a bionic knee to avoid obstacles while walking. We determined that this advantage may be attributable to their reconstructed residual knee muscles, the improved control-signal quality from the permanently implanted electrodes within their muscles, and the lack of prosthetic socket interference. Our testing also developed a versatile prosthetic knee controller capable of computationally mirroring the user’s phantom limb movements when utilized by the osseointegrated mechanoneural prosthesis. Furthermore, we demonstrated that increasing anatomical prosthetic integration in subjects with preexisting amputation can enhance prosthetic embodiment, uncovering a self-perceptive metric to more thoroughly assess the outcomes of amputation design and prosthesis selection.
CONCLUSION
Our results suggest that increased prosthetic integration at the above-knee amputation level can yield improved signaling and joint control in certain physical tasks. We posit that the capabilities demonstrated by the osseointegrated myoneural prosthesis provide evidence that further anatomical integration with the prosthesis, rather than the maintenance of an artificial separation, is required for advancing progress toward physiological function, embodiment, and rehabilitative potential after limb loss.
 Facilitating information and energy exchange through a vertically integrated bionic amputation platform.
Facilitating information and energy exchange through a vertically integrated bionic amputation platform.
The osseointegrated mechanoneural prosthesis sustains continuous knee control from motor intent originating in the central nervous system. A stylized posterior-lateral view of the transfemoral residuum reveals an agonist-antagonist myoneural interface created from residual knee extensor and flexor muscles.
Abstract
Lower-extremity prostheses have evolved through mechanical redesigns that prioritize improved cyclic locomotion. However, this limited approach to limb restoration has precluded necessary progress toward recovering the versatile acyclic movements that constitute the remainder of human athleticism. We present an osseointegrated mechanoneural prosthesis that incorporates modified hard and soft tissues along with permanently implanted hardware in a neuroembodied design. We developed a biomimetic coupling between neuromuscular signaling and joint movement that exceeds the versatility of established control methods, which depend upon conventional amputation musculature and surface electromyography. Our findings also reveal that superior residual neuromuscular function can enable prosthetic movement speeds surpassing that of intact physiology. Anatomical prosthetic integration may be necessary for meeting, and possibly exceeding, the movement capabilities of an intact limb.


