2025-08-01 マサチューセッツ工科大学(MIT)
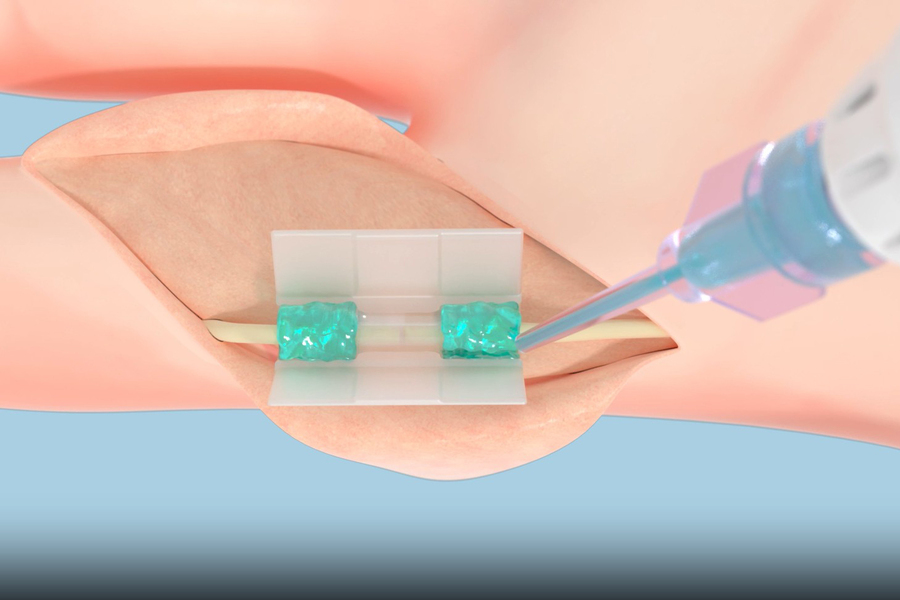
Tissium’s platform consists of biocompatible polymers (the blue, gel-like substance in this rendering) that conform to tissues and a 3D-printed chamber (clear tube at center). Credit: Courtesy of Tissium
<関連情報>
- https://news.mit.edu/2025/ushering-new-era-suture-free-tissue-reconstruction-better-healing-0801
- https://journals.lww.com/prsgo/fulltext/2024/07000/comparative_effectiveness_systematic_review_and.28.aspx
- https://www.science.org/doi/10.1126/scitranslmed.3006557
末梢神経修復における直接修復法とコネクタ補助修復法の比較有効性に関する系統的レビューとメタ解析 Comparative Effectiveness Systematic Review and Meta-analysis of Peripheral Nerve Repair Using Direct Repair and Connector-assisted Repair
Leis, Amber MD; Smetana, Brandon S. MD; Strohl, Adam B. MD; Styron, Joseph F. MD, PhD
Plastic & Reconstructive Surgery-Global Open Published:July 2024
Abstract
Background:
This clinical literature systematic review and meta-analysis were performed to assess differences in outcomes between nerves repaired with direct repair (DR) and connector-assisted repair (CAR).
Methods:
A systematic literature review for DR and CAR was performed. Studies from 1980 through August 2023 were included if DR or CAR repairs were performed in upper extremities with nerve gaps less than 5 mm and reported sensory Medical Research Council Classification (MRCC) outcomes or equivalent. Comparative analyses were planned for meaningful recovery (MR) rate (at both S3 and S3+ or better), postsurgical neuroma, cold intolerance, altered sensation, pain, and revision rate.
Results:
There were significant differences in MR rates for CAR and DR. At the MRCC S3 threshold, 96.1% of CAR and 81.3% of DR achieved MR (P < 0.0001). At the MRCC S3+ threshold, 87.1% of CAR and 54.2% of DR achieved this higher threshold of MR (P < 0.0001). There were no differences in neuroma rate or pain scores in our dataset. Altered sensation (dysesthesia, paresthesia, hyperesthesia, or hypersensitivity) was not discussed in any CAR studies, so no analysis could be performed. The revision rate for both procedures was 0%. The proportion of patients with cold intolerance was 46.2% in the DR studies, which was significantly higher than the 10.7% of patients in the CAR group.
Conclusions:
Significantly more patients achieved sensory MR and fewer had cold intolerance when the CAR technique, instead of the DR technique, was performed to repair peripheral nerve injuries.
血管および心臓奇形の最小侵襲的修復用に開発された血液抵抗性外科用接着剤 A Blood-Resistant Surgical Glue for Minimally Invasive Repair of Vessels and Heart Defects
Nora Lang, Maria J. Pereira, Yuhan Lee, Ingeborg Friehs, […] , and Pedro J. del Nido
Science Translational Medicine Published:8 Jan 2014
DOI:https://doi.org/10.1126/scitranslmed.3006557
Light-Activated Adhesive Seals Tissues
An easy way to repair vessels or attach devices to tissues would be welcomed by surgeons. An adhesive, for instance, can reconnect tissue and interface prosthetics, but currently available materials have limitations such as low strength, high toxicity, and most do not function well in wet environments. In response, Lang and colleagues developed a new biomaterial glue that is biocompatible, biodegradable, and easily manipulated. This material, called poly(glycerol sebacate acrylate) (PGSA), when combined with a photoinitiator, creates a solution that the authors called HLAA: hydrophobic light-activated adhesive. The HLAA is a thick gel that can be slathered on a tissue and then cross-linked within seconds by ultraviolet light, which is a unique feature that avoids stitches. The resulting bond is water-tight yet flexible and stays intact in the face of high pressure and flowing blood. The authors first tested their material in rats, showing that the HLAA could be used to attach a polymer patch to the heart and that the HLAA alone could seal up defects in the heart wall, performing as well as sutures. Lang et al. then moved into pigs, whose hearts beat at similar rates to humans (by contrast, rats have much higher heart rates). Lang et al. showed that the light-activated adhesive could attach a patch to the interventricular septum of a pig’s beating heart and that this patch remained in place even under higher than normal heart rates (induced by adrenaline). Additionally, the HLAA alone was able to immediately close up defects in the pig carotid artery without any bleeding complications.
The light-responsive adhesive performed well in several different in vivo scenarios, suggesting its broad applicability in the clinic, at least for cardiovascular surgeries and defects. As an added bonus, components of PGSA—namely, glycerol and sebacic acid—exist in the body and are readily metabolized. It is expected that this material could be translated soon to use in people.
Abstract
Currently, there are no clinically approved surgical glues that are nontoxic, bind strongly to tissue, and work well within wet and highly dynamic environments within the body. This is especially relevant to minimally invasive surgery that is increasingly performed to reduce postoperative complications, recovery times, and patient discomfort. We describe the engineering of a bioinspired elastic and biocompatible hydrophobic light-activated adhesive (HLAA) that achieves a strong level of adhesion to wet tissue and is not compromised by preexposure to blood. The HLAA provided an on-demand hemostatic seal, within seconds of light application, when applied to high-pressure large blood vessels and cardiac wall defects in pigs. HLAA-coated patches attached to the interventricular septum in a beating porcine heart and resisted supraphysiologic pressures by remaining attached for 24 hours, which is relevant to intracardiac interventions in humans. The HLAA could be used for many cardiovascular and surgical applications, with immediate application in repair of vascular defects and surgical hemostasis.