2025-08-15 イェール大学
<関連情報>
- https://news.yale.edu/2025/08/15/treating-brain-swelling-after-stroke-one-trial-time
- https://onlinelibrary.wiley.com/doi/10.1002/ana.27268
- https://medibio.tiisys.com/157859/
- https://www.thelancet.com/journals/laneur/article/PIIS1474-4422(24)00425-3/abstract
- https://www.thelancet.com/journals/laneur/article/PIIS1474-4422(16)30196-X/abstract
医療的および血管内治療を受けた大規模コア脳卒中における静脈内グリブライド:CHARMランダム化臨床試験のサブグループ解析 Intravenous Glyburide in Medical and Endovascular-Treated Large-Core Stroke: A Subgroup Analysis of the CHARM Randomized Clinical Trial
W. Taylor Kimberly MD, PhD, Jeffrey L. Saver MD, Bruce C.V. Campbell MBBS, PhD, Gregory W. Albers MD, Bradley J. Molyneaux MD, PhD, H.E. Hinson MD, MCR, Marcelo Rocha MD, PhD, Shilpi Mittal MD, Stephen Bacchi MBBS, PhD, Gagan Sharma MCA, Charlotte Cordonnier MD, PhD, Thorsten Steiner MD, PhD, Kazunori Toyoda MD, PhD, Max Wintermark MD, Raul G. Nogueira MD, PhD, Sven Jacobson BSE, J. Marc Simard MD, PhD, Kevin N. Sheth MD
Annals of Neurology Published: 06 June 2025
DOI:https://doi.org/10.1002/ana.27268
Abstract
Objective
The Glibenclamide for Large Hemispheric Infarction Analyzing mRS and Mortality (CHARM) trial enrolled participants with large hemispheric infarction, randomized to a placebo or intravenous glyburide. Our objective in this post-hoc study was to evaluate the relationship between baseline stroke volume and the potential efficacy of i.v. glyburide.
Methods
Participants enrolled in CHARM and aged ≤70 years were included if a <125 ml ischemic core lesion volume was measured by computed tomography perfusion or diffusion magnetic resonance imaging. The primary endpoint was a shift analysis on the 90-day modified Rankin Scale. Independent variables included age, sex, baseline National Institutes of Health Stroke Scale, world region, tissue plasminogen activator, and endovascular thrombectomy.
Results
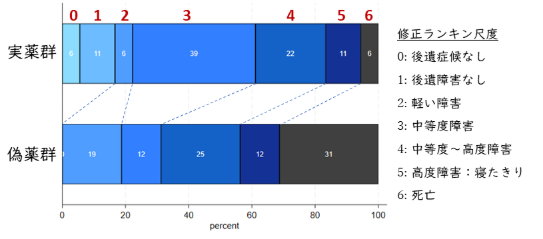
A total of 147 participants with a baseline large-core stroke volume <125 ml were available for this analysis (mean age 58 years, 37% women, baseline National Institutes of Health Stroke Scale 18, and the time to study drug was 9.1 ± 2.1 h). The median baseline core volume of 92 mL (IQR 81–107 ml) did not differ by treatment arm. The i.v. glyburide-treated participants had a favorable shift in outcome relative to the placebo (adjusted common odds ratio 2.11, 95% CI 1.10–4.01, p = 0.02). In patients who underwent endovascular thrombectomy, i.v. glyburide-treated subjects had a favorable outcome (adjusted common odds ratio 8.19, 95% CI 1.60–42.0, p = 0.01), fewer decompressive craniectomies (0% vs 25%, P = 0.02), less midline shift (4.2 ± 2.5 mm vs 7.6 ± 5.5 mm, p = 0.02), and lower 90-day mortality (5.6% vs 31%, p = 0.05) compared with the placebo, respectively.
Interpretation
This post-hoc analysis of the CHARM trial provides hypothesis-generating evidence that i.v. glyburide may improve outcome in large-core stroke <125 ml, especially among endovascular thrombectomy-treated patients. These results require confirmation in a prospective randomized clinical trial. ANN NEUROL 2025
大規模半球性脳卒中後の脳浮腫に対する静脈内グリベンクラミド(CHARM):第3相、二重盲検、プラセボ対照、ランダム化試験 Intravenous glibenclamide for cerebral oedema after large hemispheric stroke (CHARM): a phase 3, double-blind, placebo-controlled, randomised trial
Prof Kevin N Sheth, MD ∙ Prof Gregory W Albers, MD ∙ Prof Jeffrey L Saver, MD ∙ Prof Bruce C V Campbell, PhD ∙ Bradley J Molyneaux, MD ∙ Prof H E Hinson, MD ∙ et al.
The Lancet Neurology Published: December 2024
DOI:https://doi.org/10.1016/S1474-4422(24)00425-3
Summary
Background
No treatment is available to prevent brain oedema, which can occur after a large hemispheric infarction. Glibenclamide has previously been shown to improve functional outcome and reduce neurological or oedema-related death in patients younger than 70 years who were at risk of brain oedema after an acute ischaemic stroke. We aimed to assess whether intravenous glibenclamide could improve functional outcome at 90 days in patients with large hemispheric infarction.
Methods
CHARM was a phase 3, double-blind, placebo-controlled, randomised trial conducted across 143 acute stroke centres in 21 countries. We included patients aged 18–85 years with a large stroke, defined either by an Alberta Stroke Program Early CT Score (ASPECTS) of 1–5 or by an ischaemic core lesion volume of 80–300 mL on CT perfusion or MRI diffusion-weighted imaging. Patients were randomly assigned in a 1:1 ratio to either intravenous glibenclamide (8·6 mg over 72 h) or placebo. The study drug was started within 10 h of stroke onset. The primary efficacy outcome was the shift in the distribution of scores on the modified Rankin Scale at day 90, as a measure of functional outcome. The primary efficacy outcome was analysed in a modified intention-to-treat population, which included all randomly assigned patients aged 18–70 years. The safety population comprised all randomly assigned patients who received a dose. This trial is registered with ClinicalTrials.gov (NCT02864953). The trial was stopped early by the sponsor for strategic and operational reasons (slow enrolment because of COVID-19), before any unblinding or knowledge of the trial results.
Findings
Between Aug 29, 2018, and May 23, 2023, 535 patients were enrolled and randomly assigned, of whom 518 received a dose (safety population) and 431 were aged 18–70 years and comprised the modified intention-to-treat population (217 were assigned glibenclamide and 214 placebo). The mean age of patients was 58·7 (SD 9·0) years in the placebo group and 58·0 (9·5) years in the glibenclamide group; the median US National Institutes of Health Stroke Scale (NIHSS) score was 19 (IQR 16–23) in the placebo group and 19 (IQR 16–22) in the glibenclamide group; and the mean time from stroke onset to study drug start was 8·9 h (SD 2·1) in the placebo group and 9·2 h (2·1) in the glibenclamide group. Intravenous glibenclamide was not associated with a favourable shift in the modified Rankin scale at 90 days (common odds ratio [OR] 1·17 [95% CI 0·80–1·71], p=0·42). 90-day mortality was 29% (61 of 214) in the placebo group and 32% (70 of 217) in the glibenclamide group (hazard ratio 1·20 [0·85–1·70]; p=0·30). Serious adverse events in the prespecified safety population were consistent with the known safety profile of glibenclamide and included hypoglycaemia in 15 (6%) of 259 patients in the glibenclamide group and in four (2%) of 259 patients in the placebo group, leading to dose interruption or reduction in seven (3%) patients in the glibenclamide group and in one (<1%) in the placebo group.
Interpretation
Intravenous glibenclamide did not improve functional outcome in patients aged 18–70 years after large hemispheric infarction, although the trial was underpowered to make definitive conclusions because it was stopped early. Future prospective evaluation could be warranted to identify a possible benefit of intravenous glibenclamide in specific subgroups.
大規模半球性梗塞後の脳浮腫に対する静脈内グリブライドの安全性および有効性(GAMES-RP):ランダム化、二重盲検、プラセボ対照第2相試験 Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): a randomised, double-blind, placebo-controlled phase 2 trial
Dr Kevin N Sheth, MD ∙ Jordan J Elm, PhD ∙ Bradley J Molyneaux, MD ∙ Holly Hinson, MD ∙ Lauren A Beslow, MD ∙ Gordon K Sze, MD ∙ et al.
The Lancet Neurology Published: August 23, 2016
DOI:https://doi.org/10.1016/S1474-4422(16)30196-X
Summary
Background
Preclinical models of stroke have shown that intravenous glyburide reduces brain swelling and improves survival. We assessed whether intravenous glyburide (RP-1127; glibenclamide) would safely reduce brain swelling, decrease the need for decompressive craniectomy, and improve clinical outcomes in patients presenting with a large hemispheric infarction.
Methods
For this double-blind, randomised, placebo-controlled phase 2 trial, we enrolled patients (aged 18–80 years) with a clinical diagnosis of large anterior circulation hemispheric infarction for less than 10 h and baseline diffusion-weighted MRI image lesion volume of 82–300 cm3 on MRI at 18 hospitals in the USA. We used web-based randomisation (1:1) to allocate patients to the placebo or intravenous glyburide group. Intravenous glyburide was given as a 0·13 mg bolus intravenous injection for the first 2 min, followed by an infusion of 0·16 mg/h for the first 6 h and then 0·11 mg/h for the remaining 66 h. The primary efficacy outcome was the proportion of patients who achieved a modified Rankin Scale (mRS) score of 0–4 at 90 days without undergoing decompressive craniectomy. Analysis was by per protocol. Safety analysis included all randomly assigned patients who received the study drug. This trial is registered with ClinicalTrials.gov, number NCT01794182.
Findings
Between May 3, 2013, and April 30, 2015, 86 patients were randomly assigned but enrolment was stopped because of funding reasons. The funder, principal investigators, site investigators, patients, imaging core, and outcomes personnel were masked to treatment. The per-protocol study population was 41 participants who received intravenous glyburide and 36 participants who received placebo. 17 (41%) patients in the intravenous glyburide group and 14 (39%) in the placebo group had an mRS score of 0–4 at 90 days without decompressive craniectomy (adjusted odds ratio 0·87, 95% CI 0·32–2·32; p=0·77). Ten (23%) of 44 participants in the intravenous glyburide group and ten (26%) of 39 participants in the placebo group had cardiac events (p=0·76), and four of 20 had serious adverse events (two in the intravenous glyburide group and two in the placebo group, p=1·00). One cardiac death occurred in each group (p=1·00).
Interpretation
Intravenous glyburide was well tolerated in patients with large hemispheric stroke at risk for cerebral oedema. There was no difference in the composite primary outcome. Further study is warranted to assess the potential clinical benefit of a reduction in swelling by intravenous glyburide.