2025-08-22 韓国基礎科学研究院(IBS)
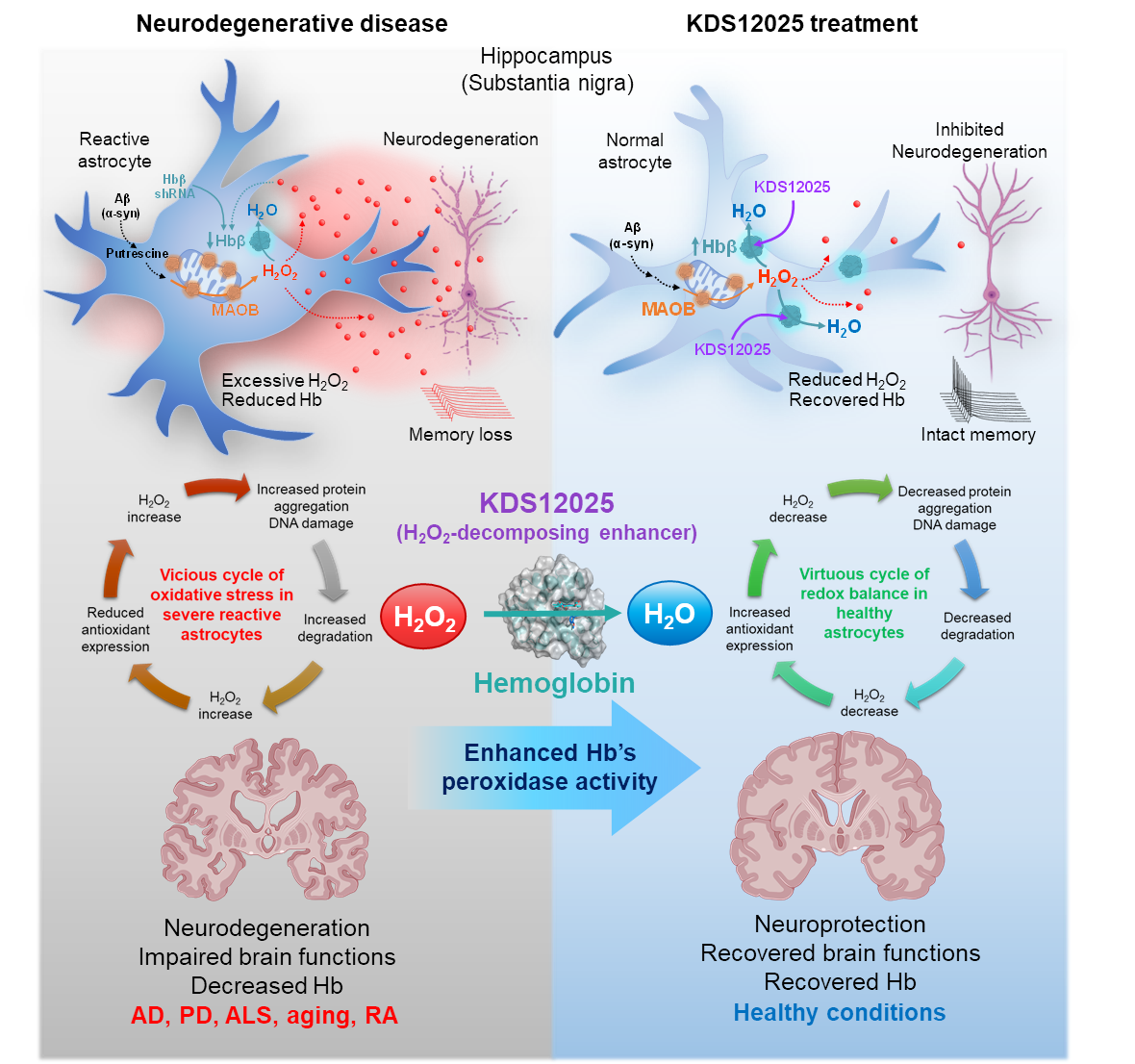
Figure 1. In neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and ALS, excessive H2O2 from reactive astrocytes triggers a vicious cycle of oxidative stress, protein aggregation, and neuronal loss. This cycle is worsened by reduced astrocytic hemoglobin, a natural antioxidant defense. KDS12025 enhances hemoglobin’s H2O2-decomposition, lowers oxidative stress, restores astrocyte and neuron health, and preserves memory and motor function—transforming a vicious cycle into a virtuous one. Beyond brain diseases, KDS12025 also shows protective effects in rheumatoid arthritis, underscoring its broad therapeutic potential.
<関連情報>
- https://www.ibs.re.kr/cop/bbs/BBSMSTR_000000000738/selectBoardArticle.do?nttId=26070&pageIndex=1&searchCnd=&searchWrd=
- https://www.nature.com/articles/s41392-025-02366-w
ヘモグロビンを偽過酸化物酵素として、酸化ストレス関連疾患の薬物標的としての役割 Hemoglobin as a pseudoperoxidase and drug target for oxidative stress-related diseases
Woojin Won,Elijah Hwejin Lee,Lizaveta Gotina,Heejung Chun,Jae-Hun Lee,Mridula Bhalla,Uiyeol Park,Daeun Kim,Tai Young Kim,Ji Won Choi,Yoowon Kim,Sun Jun Park,Jiwoon Lim,Jong-Hyun Park,Hyeon Jeong Kim,Jun Young Heo,Woosuk Chung,Myung Jin Oh,Hyun Joo An,Junghee Lee,Soo-Jin Oh,Hoon Ryu,Ae Nim Pae,Ki Duk Park & C. Justin Lee
Signal Transduction and Targeted Therapy Published:22 August 2025
DOI:https://doi.org/10.1038/s41392-025-02366-w
Abstract
Hemoglobin (Hb) is well known for transporting oxygen in the blood, but its role in the brain remains poorly understood. Here, we identified Hb in the cytosol, mitochondria, and nuclei of hippocampal and substantia nigra astrocytes and dopaminergic neurons. As a pseudoperoxidase, Hb decomposes hydrogen peroxide (H2O2) and mitigates H2O2-induced oxidative damage. However, in Alzheimer’s disease, Parkinson’s disease, and aging, excessive H2O2 diminishes astrocytic Hb, perpetuating a vicious cycle of oxidative stress and neurodegeneration. To counter the harmful effects of aberrant H2O2 production in diseases, we developed KDS12025, a BBB-permeable small molecule that enhances Hb pseudoperoxidase activity 100-fold, even at a low level of Hb. KDS12025 and its analogs achieve this enhancement through its electron-donating amine group, possibly stabilizing the complex between Hb, H2O2, and KDS12025. KDS12025 reduces astrocytic H2O2, alleviates astrogliosis, normalizes Hb, and reverts to a virtuous cycle of redox balance, preventing neurodegeneration without altering the oxygen-transport function of Hb. Gene silencing of Hb abrogates the impact of KDS12025 in both culture and animal models, confirming the necessity of Hb for the effects of KDS12025. KDS12025 extends survival and improves motor function even in severe amyotrophic lateral sclerosis and aging. Furthermore, the enrichment of astrocytic Hb in the nucleolus highlights a novel antioxidative mechanism potentially protecting against nuclear oxidative damage. Our findings suggest that Hb is a new therapeutic target for neurodegenerative diseases, with KDS12025 emerging as a first-in-class approach that enhances Hb pseudoperoxidase activity to reduce H2O2. Increasing Hb pseudoperoxidase activity with KDS12025 mitigates oxidative stress and alleviates neurodegeneration in AD, PD, and ALS patients and increases the degree of aging, with broad applicability for numerous oxidative-stress-driven diseases.