2025-08-28 ワシントン大学セントルイス校
Web要約 の発言:
Courtesy Jeffrey Brown
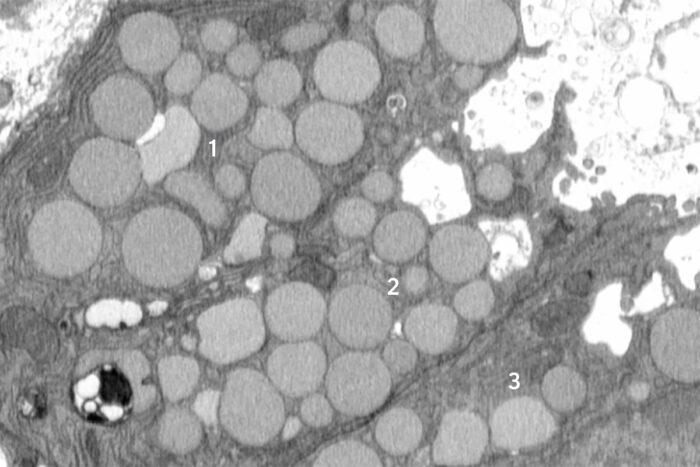
A new study from WashU Medicine identifies a previously unknown way that cells purge waste in a process that helps them revert to a stem cell-like state to promote healing after injury. Here, three mouse stomach cells (numbered) are shown jettisoning cellular debris through cavities (white) that form in their membranes. The researchers dubbed the new purging process “cathartocytosis,” combining Greek root words meaning cellular cleansing.
<関連情報>
- https://medicine.washu.edu/news/cells-vomit-waste-to-promote-healing-mouse-study-reveals/
- https://www.cell.com/cell-reports/fulltext/S2211-1247(25)00841-1
カタルトサイトーシス:分化細胞の再プログラム化過程における細胞物質の排出 Cathartocytosis: Jettisoning of cellular material during reprogramming of differentiated cells
Jeffrey W. Brown ∙ Xiaobo Lin ∙ Gabriel Anthony Nicolazzi ∙ … ∙ Megan D. Radyk, ∙ Joseph Burclaff ∙ Jason C. Mills
Cell Reports Published:July 30, 2025
DOI:https://doi.org/10.1016/j.celrep.2025.116070
Highlights
- Defining cathartocytosis, an injury-induced process cells use to downscale cellular machinery
- Three-dimensional reconstruction detailing how cells reorganize organelles during paligenosis
- Cathartocytosis and autophagy occur together in paligenosis but are mechanistically distinct
- EPG5 prevents fusion of autophagic compartments with the apical membrane after injury
Summary
Injury causes differentiated cells to undergo massive reprogramming to become proliferative and repair tissue via paligenosis. Gastric chief cells use paligenosis to reprogram into progenitor-like spasmolytic-polypeptide-expressing metaplasia (SPEM) cells. Stage 1 of paligenosis is the downscaling of mature cell architecture via a process involving lysosomes. Here, we notice that sulfated glycoproteins are not only digested during paligenosis but also excreted into the gland. Various genetic and pharmacological approaches show that endoplasmic reticulum membranes and secretory granule cargo are also excreted and that the process proceeds in parallel with but is mechanistically independent of autophagy. Three-dimensional light and electron microscopy demonstrated that excretion occurs via unique, complex, multi-chambered invaginations of the apical plasma membrane. As this lysosome-independent cell cleansing process does not seem to have been priorly described, we termed it “cathartocytosis.” Cathartocytosis allows a cell to rapidly eject excess material without waiting for autophagic and lysosomal digestion, providing for efficient cellular downscaling.