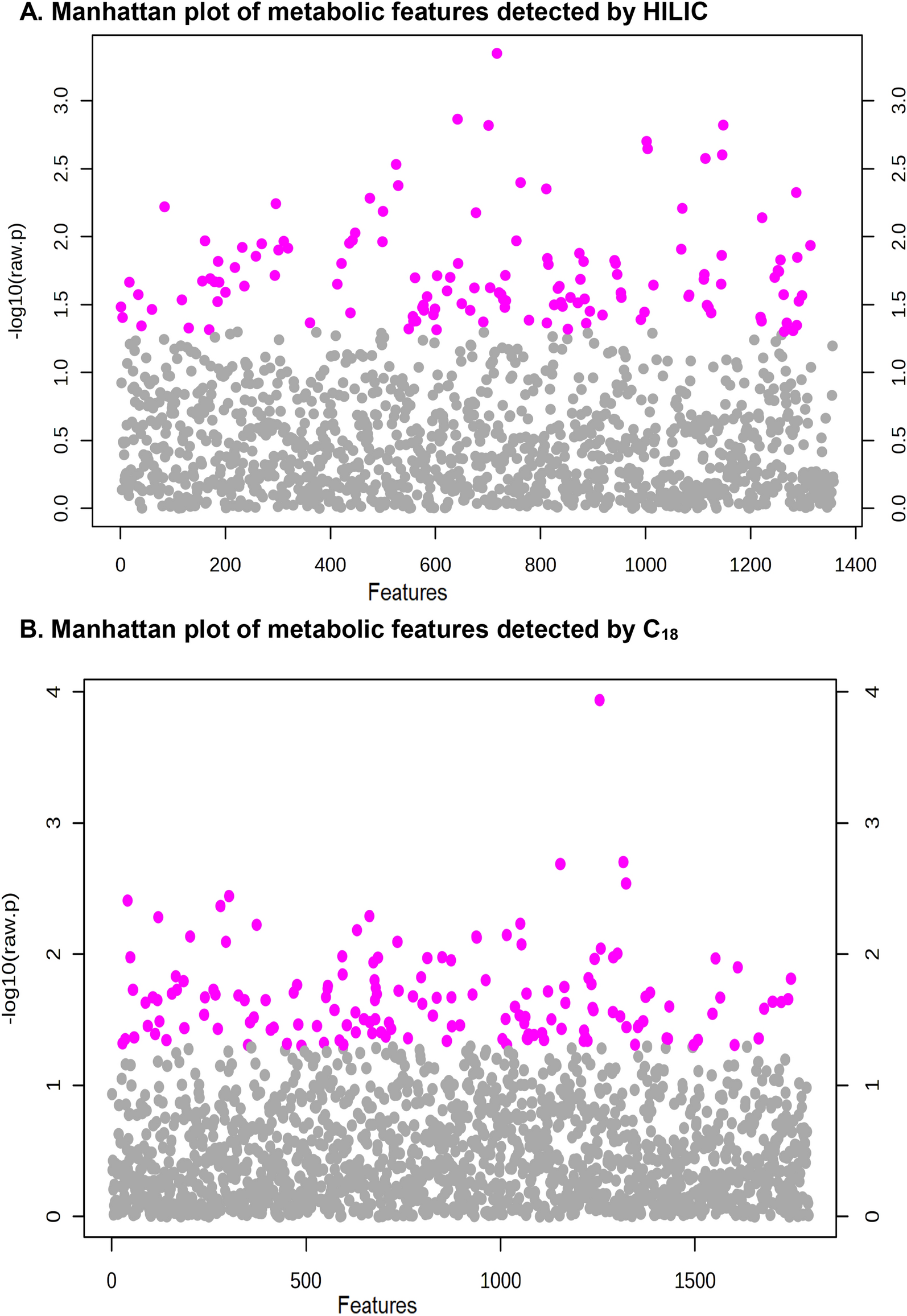
2025-10-07 ニューヨーク大学(NYU)
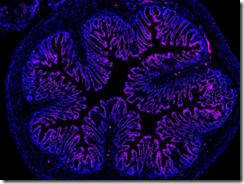
Fluorescent nanoparticles (purple) carrying a drug to treat pain accumulate in the cells of a mouse colon. Credit: Bunnett Lab
<関連情報>
- https://www.nyu.edu/about/news-publications/news/2025/october/gut-pain-microbiome-nanoparticles.html
- https://www.pnas.org/doi/10.1073/pnas.2412687122
- https://www.cell.com/cell-host-microbe/abstract/S1931-3128(25)00376-2
エンドソーム内のプロテアーゼ活性化受容体2を標的としたナノ医薬品は持続的な鎮痛効果をもたらす Nanomedicines targeting protease-activated receptor 2 in endosomes provide sustained analgesia
Shavonne L. Teng, Rocco Latorre, Divya Bhansali, +16 , and Nigel W. Bunnett
Proceedings of the National Academy of Sciences Published:October 7, 2025
DOI:https://doi.org/10.1073/pnas.2412687122
Significance
There is a pressing need to develop nonopioid treatments for pain. In the diseased colon, proteases that activate PAR2 on nociceptors and colonocytes evoke pain. Since PAR2 endosomal signaling underlies pain, PAR2 in endosomes is a relevant therapeutic target. We leveraged the predisposition of NPs to accumulate in endosomes to deliver the PAR2 antagonist, AZ3451, to intracellular sites of pain signaling. NPs that delivered AZ3451 to endosomes effectively reversed PAR2 endosomal signals, whereas unencapsulated AZ3451 was minimally effective. When administered into the colonic lumen of mice, NP-encapsulated AZ3451, but not unencapsulated AZ3451, reversed pain and normalized aberrant behavior in preclinical models of inflammatory bowel disease. Nanomedicines that block intracellular signaling of PAR2, and possibly other GPCRs, effectively relieve visceral pain.
バクテロイデス・フラギリスのプロテアーゼは宿主のPAR2を活性化し、腸の痛みと炎症を引き起こす A Bacteroides fragilis protease activates host PAR2 to induce intestinal pain and inflammation
Markus Lakemeyer ∙ Rocco Latorre ∙ Kristyna Blazkova ∙ … ∙ Alan E. Lomax ∙ Nigel W. Bunnett ∙ Matthew Bogyo
Cell Host & Microbe Published:September 26, 2025
DOI:https://doi.org/10.1016/j.chom.2025.09.010
Highlights
- Intestinal bacteria secrete proteases that affect host PAR2 signaling
- Chemoproteomics identifies Bfp1, a serine protease released by Bacteroides fragilis
- Bfp1 activates PAR2 to disrupt epithelial integrity, induce nociception, and inflammation
- The Bfp1-PAR2 axis links the microbiota to pain and inflammation in the gut
Summary
Protease-activated receptor 2 (PAR2) is a central regulator of intestinal barrier function, inflammation, and pain. Upregulated intestinal proteolysis and PAR2 signaling are implicated in inflammatory bowel diseases (IBDs) and irritable bowel syndrome (IBS), conditions often associated with gut microbiome alterations. To identify potential bacterial regulators of PAR2 activity, we developed a functional assay for PAR2 processing to screen a library of diverse gut microbes. We identify multiple bacteria that secrete proteases capable of cleaving host PAR2. Using chemoproteomic profiling with a covalent irreversible inhibitor, we uncovered a previously uncharacterized Bacteroides fragilis serine protease 1 (Bfp1) and show that it cleaves and activates PAR2 in multicellular and murine models. PAR2 cleavage by Bfp1 disrupts the intestinal barrier, sensitizes nociceptors, and triggers colonic inflammation and abdominal pain. Collectively, our findings uncover Bfp1-mediated PAR2 processing as an axis of host-commensal interaction in the gut that has the potential to be targeted for therapeutic intervention in IBD or IBS.