2025-11-03 ゲーテ大学
<関連情報>
- https://aktuelles.uni-frankfurt.de/english/new-study-shows-pomegranate-derived-compound-strengthens-immune-defense/
- https://www.nature.com/articles/s43587-025-00996-x
- https://www.cell.com/immunity/fulltext/S1074-7613(22)00508-8
マイトファジー誘導剤ウロリチンAの加齢性免疫低下に対する効果:ランダム化プラセボ対照試験 Effect of the mitophagy inducer urolithin A on age-related immune decline: a randomized, placebo-controlled trial
Dominic Denk,Anurag Singh,Herbert G. Kasler,Davide D’Amico,Julia Rey,Lucía Alcober-Boquet,Johanna M. Gorol,Christoph Steup,Ritesh Tiwari,Ryan Kwok,Rafael J. Argüello,Julie Faitg,Kathrin Sprinzl,Stefan Zeuzem,Valentina Nekljudova,Sibylle Loibl,Eric Verdin,Chris Rinsch & Florian R. Greten
Nature Aging Published:31 October 2025
DOI:https://doi.org/10.1038/s43587-025-00996-x
Abstract
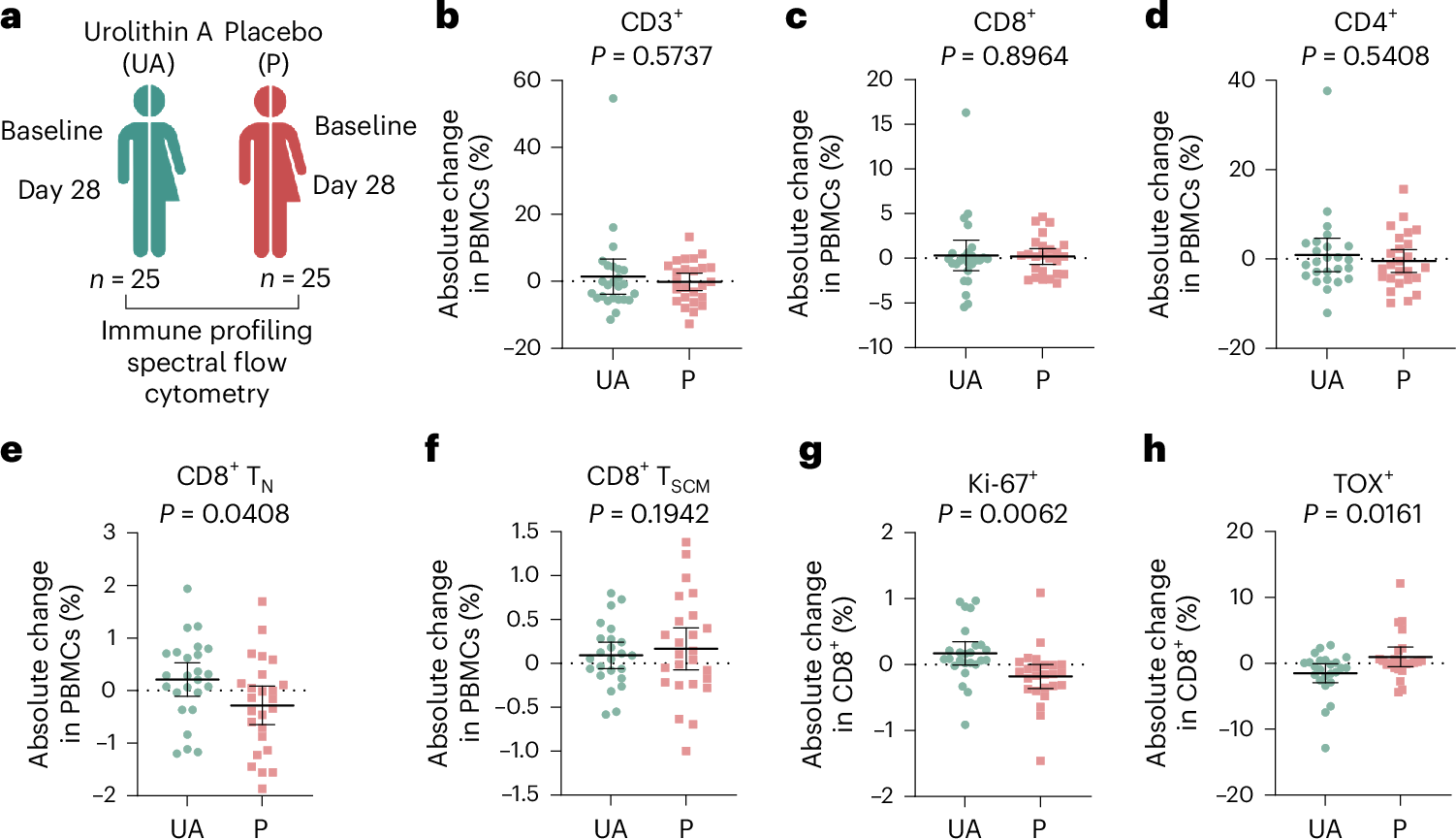
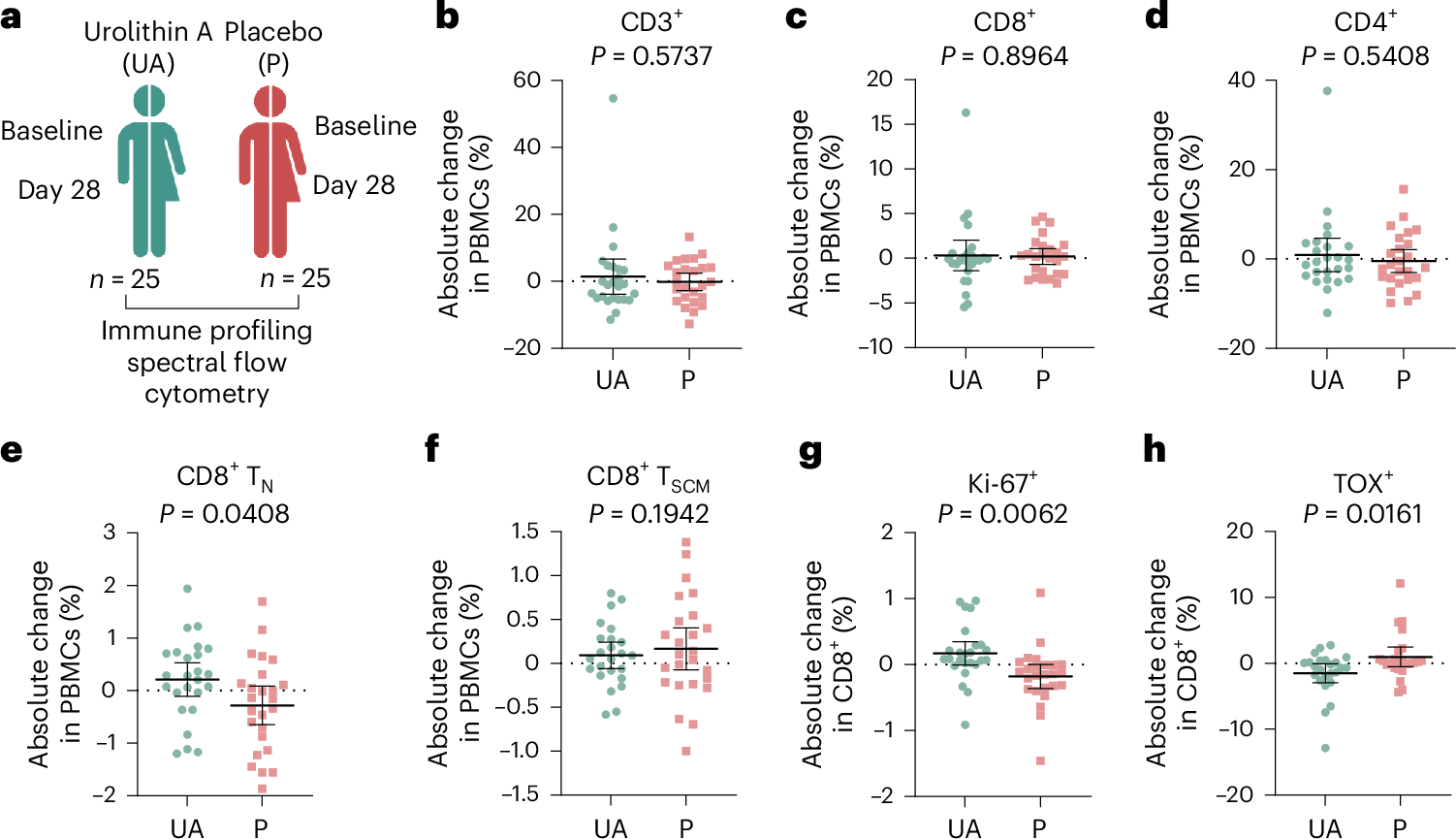
Mitochondrial dysfunction and stem cell exhaustion contribute to age-related immune decline, yet clinical interventions targeting immune aging are lacking. Recently, we demonstrated that urolithin A (UA), a mitophagy inducer, expands T memory stem cells (TSCM) and naive T cells in mice. In this randomized, double-blind, placebo-controlled trial, 50 healthy middle-aged adults received oral UA (1,000 mg day-1) or placebo for 4 weeks; time points of analysis were baseline and day 28. Primary outcomes were phenotypical changes in peripheral CD3+ T cell subsets and immune metabolic remodeling. UA expanded peripheral naive-like, less terminally exhausted CD8+ cells (treatment difference 0.50 percentage points; 95% CI = 0.16 to 0.83; P = 0.0437) while also increasing CD8+ fatty acid oxidation capacity (treatment difference = 14.72 percentage points; 95% confidence interval (CI) = 6.46 to 22.99; P = 0.0061). Secondary outcomes included changes in plasma cytokine levels (IL-6, TNF, IL-1β, IL-10), immune populations assessed via flow cytometry, immune cell function, and mitochondrial content. Analysis revealed augmented mitochondrial biogenesis in CD8+ cells, increased peripheral CD56dimCD16bright NK cells, and nonclassical CD14loCD16hi monocytes in UA-treated participants, as well as improved activation-elicited TNF secretion in T cells and bacterial uptake by monocytes. Exploratory single-cell RNA sequencing demonstrated UA-driven transcriptional shifts across immune populations, modulating pathways linked to inflammation and metabolism. These findings indicate that short-term UA supplementation modulates human immune cell composition and function, supporting its potential to counteract age-related immune decline and inflammaging. ClinicalTrials.gov registration number: NCT05735886.
ウロリチンA誘導マイトファジーによる優れた抗腫瘍免疫力を持つT記憶幹細胞の増殖 Expansion of T memory stem cells with superior anti-tumor immunity by Urolithin A-induced mitophagy
Dominic Denk ∙ Valentina Petrocelli ∙ Claire Conche ∙ … ∙ Pénélope A. Andreux ∙ Chris Rinsch ∙ Florian R. Greten
Immunity Published:October 18, 2022
DOI:https://doi.org/10.1016/j.immuni.2022.09.014
Highlights
- UA induces Pink1-dependent mitophagy in CD8+ cells causing PGAM5 release
- Cytosolic PGAM5 augments Wnt signaling to drive TSCM formation
- TSCM induction augments anti-tumor immunity in vivo
- Urolithin can be used for ex vivo expansion of CAR-expressing TSCM

Summary
T memory stem cells (TSCM) display increased self-renewal and prolonged survival capabilities, thus preventing T cell exhaustion and promoting effective anti-tumor T cell responses. TSCM cells can be expanded by Urolithin A (UA), which is produced by the commensal gut microbiome from foods rich in ellagitannins and is known to improve mitochondrial health. Oral UA administration to tumor-bearing mice conferred strong anti-tumor CD8+ T cell immunity, whereas ex vivo UA pre-treated T cells displayed improved anti-tumor function upon adoptive cell transfer. UA-induced TSCM formation depended on Pink1-mediated mitophagy triggering cytosolic release of the mitochondrial phosphatase Pgam5. Cytosolic Pgam5 dephosphorylated β-catenin, which drove Wnt signaling and compensatory mitochondrial biogenesis. Collectively, we unravel a critical signaling pathway linking mitophagy to TSCM formation and suggest that the well-tolerated metabolic compound UA represents an attractive option to improve immune therapy.