2026-02-09 マウントサイナイ医療システム(MSHS)
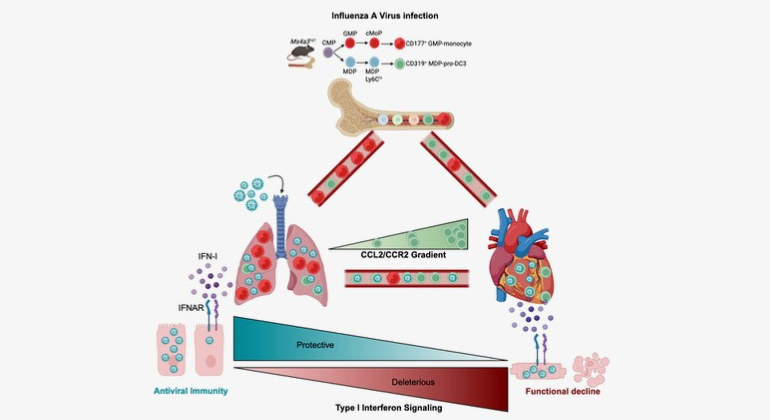
Graphical Abstract summarizing the key findings of the paper. The authors found that severe influenza damages the heart by exploiting a specific immune cells and engaging a type-I interferon response. The authors also show that therapeutic silencing of the response mitigates heart damage.
<関連情報>
- https://www.mountsinai.org/about/newsroom/2026/mount-sinai-scientists-uncover-link-between-influenza-and-heart-disease
- https://www.cell.com/immunity/abstract/S1074-7613(25)00567-9
インフルエンザは骨髄細胞を乗っ取り、I型インターフェロンを燃料とした心臓損傷を引き起こす Influenza hijacks myeloid cells to inflict type-I interferon-fueled damage in the heart
Jeffrey Downey ∙ Ana Oliveira-Coelho ∙ Máté G. Kiss ∙ … ∙ Mandy M.T. van Leent, ∙ Michael Schotsaert ∙ Filip K. Swirski
Immunity Published:February 9, 2026
DOI:https://doi.org/10.1016/j.immuni.2025.12.011
Highlights
- Severe IAV infection imparts a long-lasting decline in cardiac function
- Atypical GMP-independent CD319+ pro-DC3s transport infectious IAV to cardiomyocytes
- Direct IFN-I signaling on cardiomyocytes drives cardiac dysfunction
- mod-mRNA delivery of dominant-negative IFNAR1 on cardiomyocytes ameliorates damage
Summary
Abundant evidence has correlated influenza infection with cardiovascular disease, yet mechanisms linking infection with the heart remain poorly understood. Here, we show that influenza infection damaged the human and murine heart. In mice, we showed that shortly after pulmonary infection, the virus infected a circulating myeloid pro-dendritic cell 3 (pro-DC3) that expressed high concentrations of the chemokine receptor CCR2. The heart, which produces abundant CCL2, preferentially attracted infected pro-DC3. In the myocardium, the virus escaped pro-DC3, infected cardiomyocytes, and triggered production of type-I interferon (IFN-I). Engagement of the IFN-I receptor (IFNAR1) on cardiomyocytes caused tissue damage and compromised heart function. Genetically and therapeutically dampening IFNAR1 exclusively in cardiomyocytes protected the heart while preserving anti-viral immunity in the lung. Our results identify a series of host-pathogen interactions that propagate tissue damage and uncover an axis for intervention to mitigate cardiovascular risk following viral infection.