アメリカ大陸で数千万人が罹患しているが、有効な治療法がない。 The condition affects tens of millions across the Americas but lacks effective treatments
2022-09-08 ジョージア大学 (UGA)
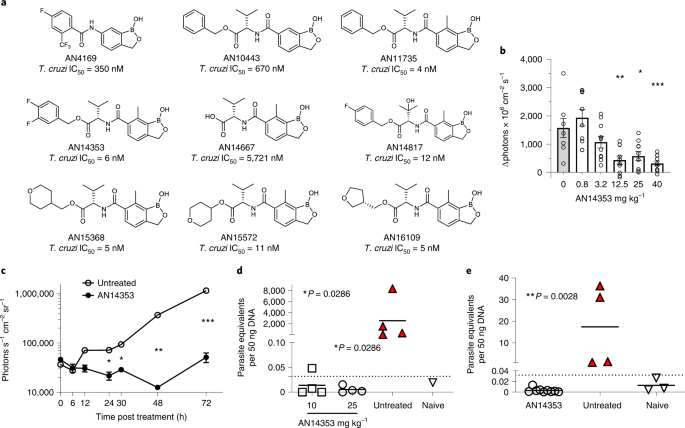
この薬剤は、AN15368として知られる抗寄生虫化合物で、今後数年のうちにヒトでの臨床試験が開始される見込みである。
新薬は、病気の原因となる寄生虫、Trypanosoma cruzi、別名T.cruziをターゲットにすることで作用する。
Nature Microbiology誌に掲載されたこの研究では、テキサス州の研究施設で寄生虫に自然感染したマウスと非ヒト霊長類に、新薬が100%有効であることが判明した。また、動物たちはこの薬にさらされたことによる重大な副作用はなかった。
<関連情報>
- https://news.uga.edu/researchers-discover-potential-treatment-for-chagas-disease/
- https://www.nature.com/articles/s41564-022-01211-y
霊長類におけるシャーガス病治療に有効な経口活性型Benzoxaboroleプロドラッグの創製.Discovery of an orally active benzoxaborole prodrug effective in the treatment of Chagas disease in non-human primates
Angel M. Padilla,Wei Wang,Tsutomu Akama,David S. Carter,Eric Easom,Yvonne Freund,Jason S. Halladay,Yang Liu,Sarah A. Hamer,Carolyn L. Hodo,Gregory K. Wilkerson,Dylan Orr,Brooke White,Arlene George,Huifeng Shen,Yiru Jin,Michael Zhuo Wang,Susanna Tse,Robert T. Jacobs & Rick L. Tarleton
Nature Microbiology Published:05 September 2022
DOI:https://doi.org/10.1038/s41564-022-01211-y
Abstract
Trypanosoma cruzi, the agent of Chagas disease, probably infects tens of millions of people, primarily in Latin America, causing morbidity and mortality. The options for treatment and prevention of Chagas disease are limited and underutilized. Here we describe the discovery of a series of benzoxaborole compounds with nanomolar activity against extra- and intracellular stages of T. cruzi. Leveraging both ongoing drug discovery efforts in related kinetoplastids, and the exceptional models for rapid drug screening and optimization in T. cruzi, we have identified the prodrug AN15368 that is activated by parasite carboxypeptidases to yield a compound that targets the messenger RNA processing pathway in T. cruzi. AN15368 was found to be active in vitro and in vivo against a range of genetically distinct T. cruzi lineages and was uniformly curative in non-human primates (NHPs) with long-term naturally acquired infections. Treatment in NHPs also revealed no detectable acute toxicity or long-term health or reproductive impact. Thus, AN15368 is an extensively validated and apparently safe, clinically ready candidate with promising potential for prevention and treatment of Chagas disease.


