2023-06-12 ワシントン大学セントルイス校
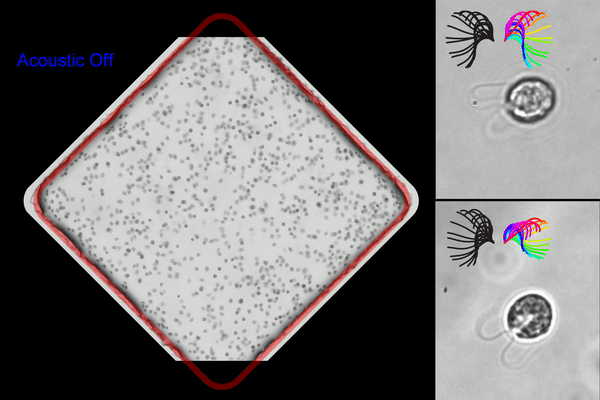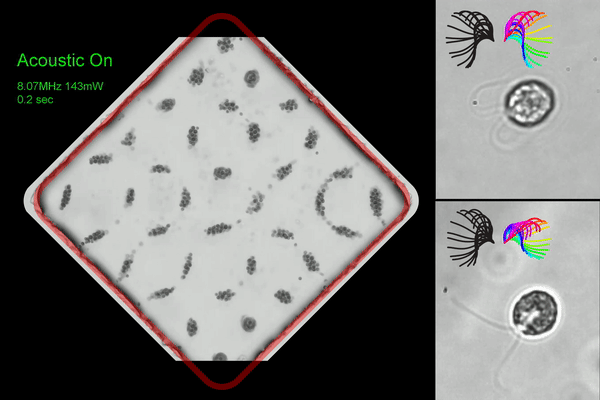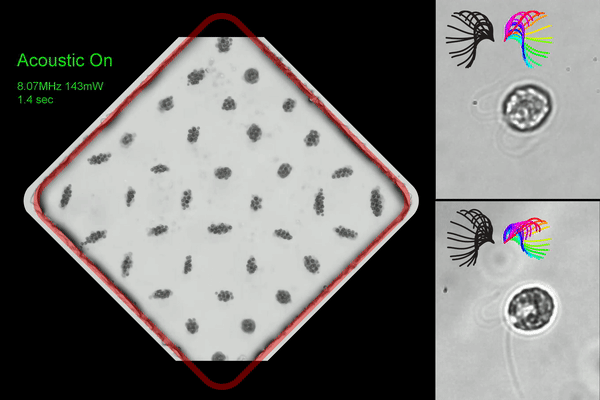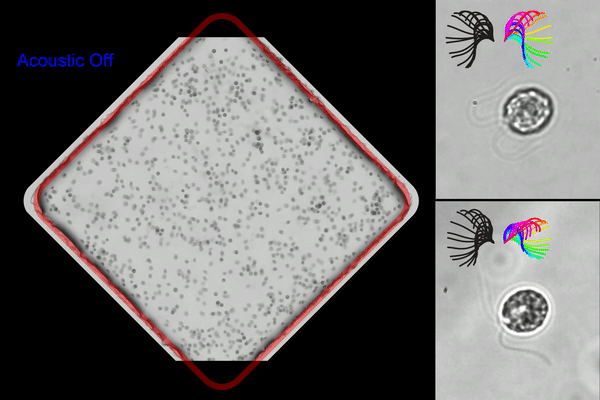
Washington University researchers created an acoustic microfluidic method that offers new opportunities to conduct experiments with swimming cells and microorganisms. This image shows acoustic trapping and releasing (left), and trapped cells with synchronous (right top) and asynchronous cilia waveform (right bottom). (Courtesy of Meacham lab/Washington University)
◆研究では、まぶたのような微小な毛様構造で流体を動かし、細胞を推進するモデル生物である単細胞藻クラミドモナス・ラインハルティを使用しました。この手法は、音響波の組み合わせを使用して細胞を捕捉し、ガラスのマイクロチャンネルを使用して表面音響波を体積音響波に変換することで効率を改善しました。この手法により、水泳する細胞を捕捉しながら回転を制約することなく研究できるようになりました。また、複数のトラップを同時に作成し、並行して捕捉した細胞の分析を行うことが可能です。
◆この手法は、細胞の運動性を理解する上で重要であり、さまざまな未解決の生物学的な問いに取り組むための制御された環境を提供します。
<関連情報>
- https://source.wustl.edu/2023/06/treadmill-for-microswimmers-allows-closer-look-at-behavior/
- https://www.pnas.org/doi/10.1073/pnas.2218951120
単細胞マイクロスイマーの堅牢な音響トラッピングと摂動により、3次元的な泳ぎと毛様体協調が明らかになった。 Robust acoustic trapping and perturbation of single-cell microswimmers illuminate three-dimensional swimming and ciliary coordination
Mingyang Cui, Susan K. , Philip V. Bayly, and J. Mark Meacham
Proceedings of the National Academy of Sciences Published:June 12, 2023
DOI:https://doi.org/10.1073/pnas.2218951120
Significance
Acoustic trapping and manipulation of single-cell microswimmers was achieved using ultrasonic standing waves in microfluidic chambers. Bulk acoustic waves produced high trapping forces at low input power, while high-frequency, short-wavelength surface acoustic wave actuation provided the high temporal (milliseconds) and spatial (submicron) resolution needed to characterize three-dimensional body motion and cilia waveform of swimming Chlamydomonas reinhardtii cells. Critically, noncontact acoustic trapping did not constrain body rotation or cilia motion. Studies using our hybrid acoustic tweezers revealed effects of temperature and viscosity on swimming characteristics and cilia beating, as well as remarkable robustness of beating to mechanical perturbation. The ability to acoustically trap and perturb strongly swimming microorganisms can be transformative in numerous applications in biology, biomedicine, and biophysics.
Abstract
We report a label-free acoustic microfluidic method to confine single, cilia-driven swimming cells in space without limiting their rotational degrees of freedom. Our platform integrates a surface acoustic wave (SAW) actuator and bulk acoustic wave (BAW) trapping array to enable multiplexed analysis with high spatial resolution and trapping forces that are strong enough to hold individual microswimmers. The hybrid BAW/SAW acoustic tweezers employ high-efficiency mode conversion to achieve submicron image resolution while compensating for parasitic system losses to immersion oil in contact with the microfluidic chip. We use the platform to quantify cilia and cell body motion for wildtype biciliate cells, investigating effects of environmental variables like temperature and viscosity on ciliary beating, synchronization, and three-dimensional helical swimming. We confirm and expand upon the existing understanding of these phenomena, for example determining that increasing viscosity promotes asynchronous beating. Motile cilia are subcellular organelles that propel microorganisms or direct fluid and particulate flow. Thus, cilia are critical to cell survival and human health. The unicellular alga Chlamydomonas reinhardtii is widely used to investigate the mechanisms underlying ciliary beating and coordination. However, freely swimming cells are difficult to image with sufficient resolution to capture cilia motion, necessitating that the cell body be held during experiments. Acoustic confinement is a compelling alternative to use of a micropipette, or to magnetic, electrical, and optical trapping that may modify the cells and affect their behavior. Beyond establishing our approach to studying microswimmers, we demonstrate a unique ability to mechanically perturb cells via rapid acoustic positioning.