2023-06-14 ヒューストン大学(UH)
◆このツールは、T細胞ががん細胞ではなく心臓のタンパク質を標的とし、深刻なダメージを与えた事例を特定することに成功しました。CrossDomeは、さまざまな要因を分析することで、T細胞療法の安全性に関する洞察を提供し、研究者がより安全な治療法を開発できるようにします。
◆このツールは、異なるデータセットを組み合わせて、遺伝子発現や免疫反応のトリガーを評価します。技術者向けの高度な制御と、非技術者向けの使いやすいインターフェースの両方を提供します。
<関連情報>
- https://uh.edu/news-events/stories/2023/june-2023/006142023-altunes-immunotherapy-safety-crossdome.php
- https://www.frontiersin.org/articles/10.3389/fimmu.2023.1142573/full
CrossDome:免疫ペプチドミクスデータベースを用いて交差反応リスクを予測する対話型Rパッケージ
CrossDome: an interactive R package to predict cross-reactivity risk using immunopeptidomics databases
Andre F. Fonseca and Dinler A. Antunes
Frontiers in Immunology Published:12 June 2023
DOI:https://doi.org/10.3389/fimmu.2023.1142573
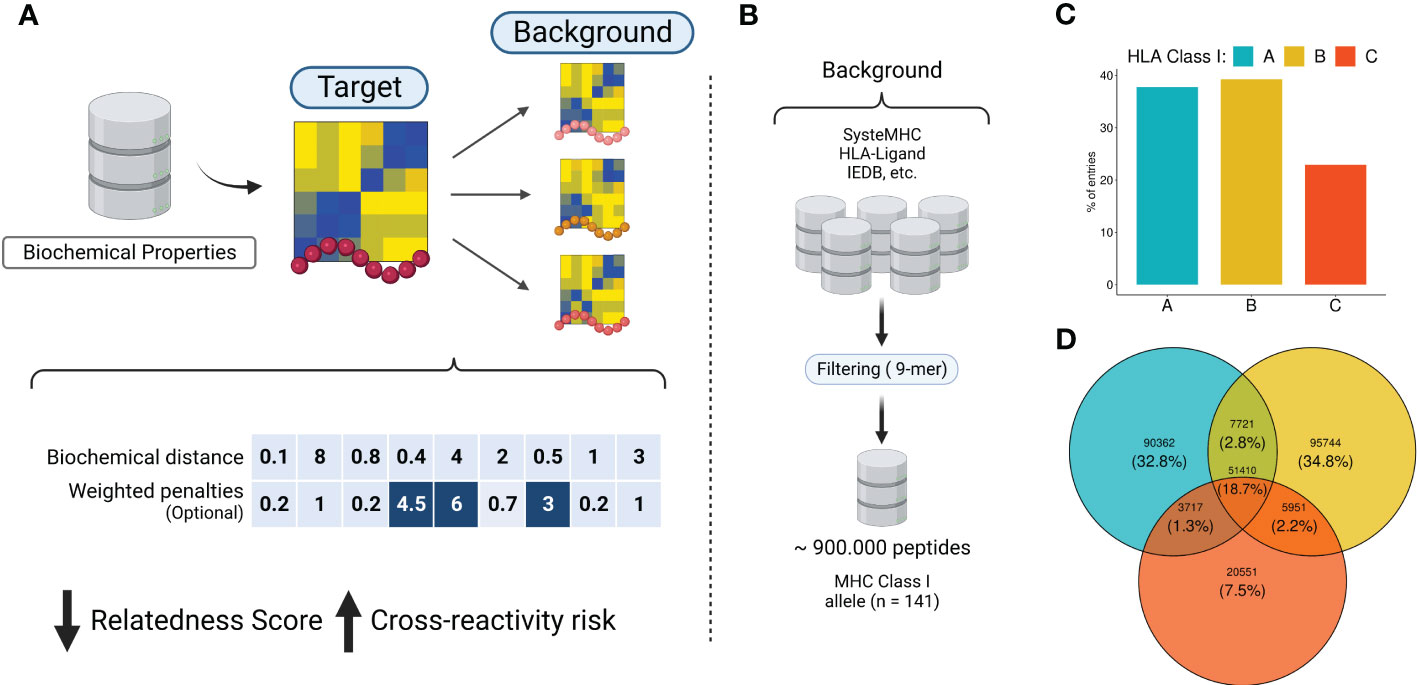
T-cell-based immunotherapies hold tremendous potential in the fight against cancer, thanks to their capacity to specifically targeting diseased cells. Nevertheless, this potential has been tempered with safety concerns regarding the possible recognition of unknown off-targets displayed by healthy cells. In a notorious example, engineered T-cells specific to MAGEA3 (EVDPIGHLY) also recognized a TITIN-derived peptide (ESDPIVAQY) expressed by cardiac cells, inducing lethal damage in melanoma patients. Such off-target toxicity has been related to T-cell cross-reactivity induced by molecular mimicry. In this context, there is growing interest in developing the means to avoid off-target toxicity, and to provide safer immunotherapy products. To this end, we present CrossDome, a multi-omics suite to predict the off-target toxicity risk of T-cell-based immunotherapies. Our suite provides two alternative protocols, i) a peptide-centered prediction, or ii) a TCR-centered prediction. As proof-of-principle, we evaluate our approach using 16 well-known cross-reactivity cases involving cancer-associated antigens. With CrossDome, the TITIN-derived peptide was predicted at the 99+ percentile rank among 36,000 scored candidates (p-value < 0.001). In addition, off-targets for all the 16 known cases were predicted within the top ranges of relatedness score on a Monte Carlo simulation with over 5 million putative peptide pairs, allowing us to determine a cut-off p-value for off-target toxicity risk. We also implemented a penalty system based on TCR hotspots, named contact map (CM). This TCR-centered approach improved upon the peptide-centered prediction on the MAGEA3-TITIN screening (e.g., from 27th to 6th, out of 36,000 ranked peptides). Next, we used an extended dataset of experimentally-determined cross-reactive peptides to evaluate alternative CrossDome protocols. The level of enrichment of validated cases among top 50 best-scored peptides was 63% for the peptide-centered protocol, and up to 82% for the TCR-centered protocol. Finally, we performed functional characterization of top ranking candidates, by integrating expression data, HLA binding, and immunogenicity predictions. CrossDome was designed as an R package for easy integration with antigen discovery pipelines, and an interactive web interface for users without coding experience. CrossDome is under active development, and it is available at https://github.com/AntunesLab/crossdome.