2023-08-14 オークリッジ国立研究所(ORNL)
◆この研究は、肺、結腸、乳房、膵臓、前立腺がんなど、高度に侵略的な腫瘍形成が見られるがん種の治療に有望なアプローチとされています。
<関連情報>
- https://www.ornl.gov/news/neutrons-seek-stop-cancer-hijacking-metabolic-highway
- https://www.nature.com/articles/s42004-023-00964-9
室温X線・中性子結晶構造解析でセリンヒドロキシメチル基転移酵素のプロトン化状態と基質追跡を解明 Revealing protonation states and tracking substrate in serine hydroxymethyltransferase with room-temperature X-ray and neutron crystallography
Victoria N. Drago,Claudia Campos,Mattea Hooper,Aliyah Collins,Oksana Gerlits,Kevin L. Weiss,Matthew P. Blakeley,Robert S. Phillips & Andrey Kovalevsky
Communications Chemistry Published:03 August 2023
DOI:https://doi.org/10.1038/s42004-023-00964-9
Abstract
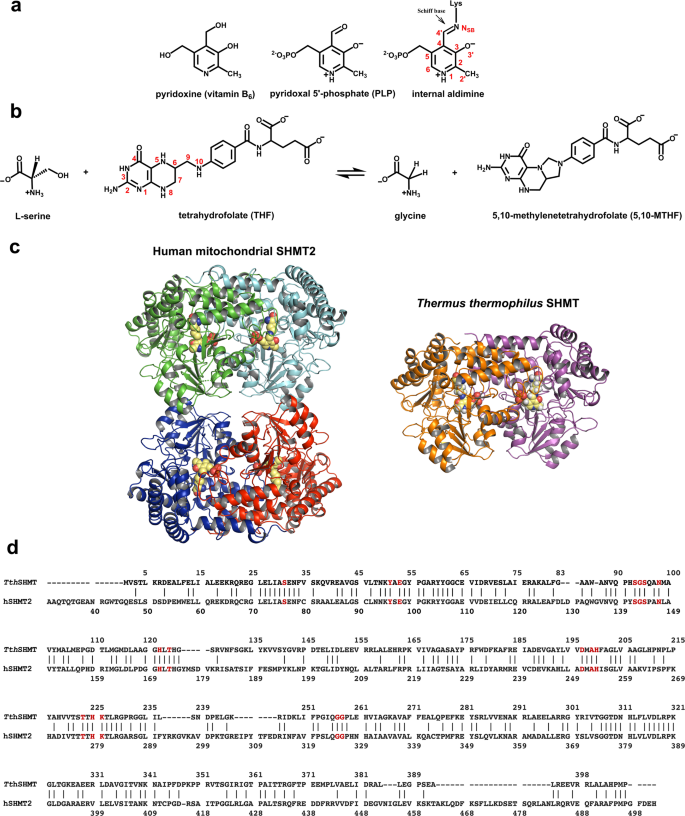
Pyridoxal 5’-phosphate (PLP)-dependent enzymes utilize a vitamin B6-derived cofactor to perform a myriad of chemical transformations on amino acids and other small molecules. Some PLP-dependent enzymes, such as serine hydroxymethyltransferase (SHMT), are promising drug targets for the design of small-molecule antimicrobials and anticancer therapeutics, while others have been used to synthesize pharmaceutical building blocks. Understanding PLP-dependent catalysis and the reaction specificity is crucial to advance structure-assisted drug design and enzyme engineering. Here we report the direct determination of the protonation states in the active site of Thermus thermophilus SHMT (TthSHMT) in the internal aldimine state using room-temperature joint X-ray/neutron crystallography. Conserved active site architecture of the model enzyme TthSHMT and of human mitochondrial SHMT (hSHMT2) were compared by obtaining a room-temperature X-ray structure of hSHMT2, suggesting identical protonation states in the human enzyme. The amino acid substrate serine pathway through the TthSHMT active site cavity was tracked, revealing the peripheral and cationic binding sites that correspond to the pre-Michaelis and pseudo-Michaelis complexes, respectively. At the peripheral binding site, the substrate is bound in the zwitterionic form. By analyzing the observed protonation states, Glu53, but not His residues, is proposed as the general base catalyst, orchestrating the retro-aldol transformation of L-serine into glycine.