2023-08-24 ハーバード大学
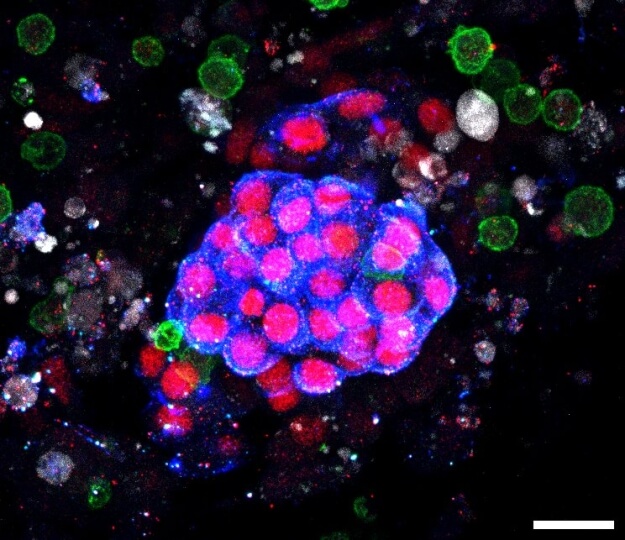
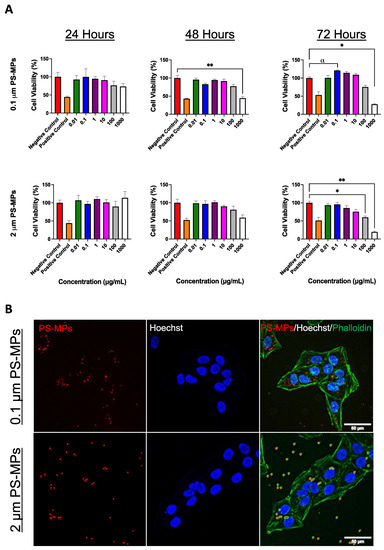
The team’s study demonstrated in a novel immune-infiltrated human kidney organoid-on-chip model that a T cell bispecific antibody (TCB) targeting an antigen from the Wilms tumor-1 protein (WT1-TCB) specifically recruits immune cells, including cytotoxic T cells (shown in green), to clusters of podocytes (shown in blue), leading to their destruction. The grey staining is derived from dying cells. Credit: Wyss Institute at Harvard University
◆この問題を解決するために、ハーバード大学とその関連機関の研究チームが、免疫浸潤した腎臓組織モデルを開発し、TCBなどの免疫療法薬の標的外、腫瘍内効果を評価するための貴重なツールとして利用しました。この研究により、TCBの標的外効果の評価がより効果的に行える可能性が示されました。
<関連情報>
- https://seas.harvard.edu/news/2023/08/adding-immunity-human-kidney-chip-advances-cancer-drug-testing
- https://www.pnas.org/doi/10.1073/pnas.2305322120
T細胞バイスペシフィック抗体(TCB)を評価するための免疫浸潤した腎臓組織モデル Immune-infiltrated kidney organoid-on-chip model for assessing T cell bispecific antibodies
Katharina T. Kroll,Mariana M. Mata,Kimberly A. Homan,Virginie Micallef,Alejandro Carpy,Ken Hiratsuka,Ryuji Morizane,Annie Moisan,Marcel Gubler,Antje-Christine Walz,Estelle Marrer-Berger, and Jennifer A. Lewis
Proceedings of the National Academy of Sciences Published:August 21, 2023
DOI:https://doi.org/10.1073/pnas.2305322120
Significance
Human models that recapitulate the cellular diversity, 3 dimensional architecture, and physiological function of native tissues are needed to evaluate emerging cancer immuno-therapeutics, such as T cell bispecific antibodies (TCBs). One target of substantial interest is Wilms Tumor 1 (WT1), a highly validated tumor antigen expressed in multiple leukemias and solid tumors as well as podocytes present in human kidneys. To assess their “off-tumor, on-target” effects, we created an immune-infiltrated, vascularized kidney organoid-on-chip model in which TCBs and peripheral blood mononuclear cells (PBMCs) are cocirculated. Using this model, we observed rapid CD8+ T cell expansion and activation under flow accompanied by dose-dependent and selective T cell–mediated killing.
Abstract
T cell bispecific antibodies (TCBs) are the focus of intense development for cancer immunotherapy. Recently, peptide-MHC (major histocompatibility complex)-targeted TCBs have emerged as a new class of biotherapeutics with improved specificity. These TCBs simultaneously bind to target peptides presented by the polymorphic, species-specific MHC encoded by the human leukocyte antigen (HLA) allele present on target cells and to the CD3 coreceptor expressed by human T lymphocytes. Unfortunately, traditional models for assessing their effects on human tissues often lack predictive capability, particularly for “on-target, off-tumor” interactions. Here, we report an immune-infiltrated, kidney organoid-on-chip model in which peripheral blood mononuclear cells (PBMCs) along with nontargeting (control) or targeting TCB-based tool compounds are circulated under flow. The target consists of the RMF peptide derived from the intracellular tumor antigen Wilms’ tumor 1 (WT1) presented on HLA-A2 via a bivalent T cell receptor-like binding domain. Using our model, we measured TCB-mediated CD8+ T cell activation and killing of RMF-HLA-A2-presenting cells in the presence of PBMCs and multiple tool compounds. DP47, a non-pMHC-targeting TCB that only binds to CD3 (negative control), does not promote T cell activation and killing. Conversely, the nonspecific ESK1-like TCB (positive control) promotes CD8+ T cell expansion accompanied by dose-dependent T cell–mediated killing of multiple cell types, while WT1-TCB* recognizing the RMF-HLA-A2 complex with high specificity, leads solely to selective killing of WT1-expressing cells within kidney organoids under flow. Our 3D kidney organoid model offers a platform for preclinical testing of cancer immunotherapies and investigating tissue-immune system interactions.