2024-06-13 ロスアラモス国立研究所(LANL)
<関連情報>
- https://discover.lanl.gov/news/0613-covid-therapeutics/
- https://www.nature.com/articles/s41586-024-07385-1
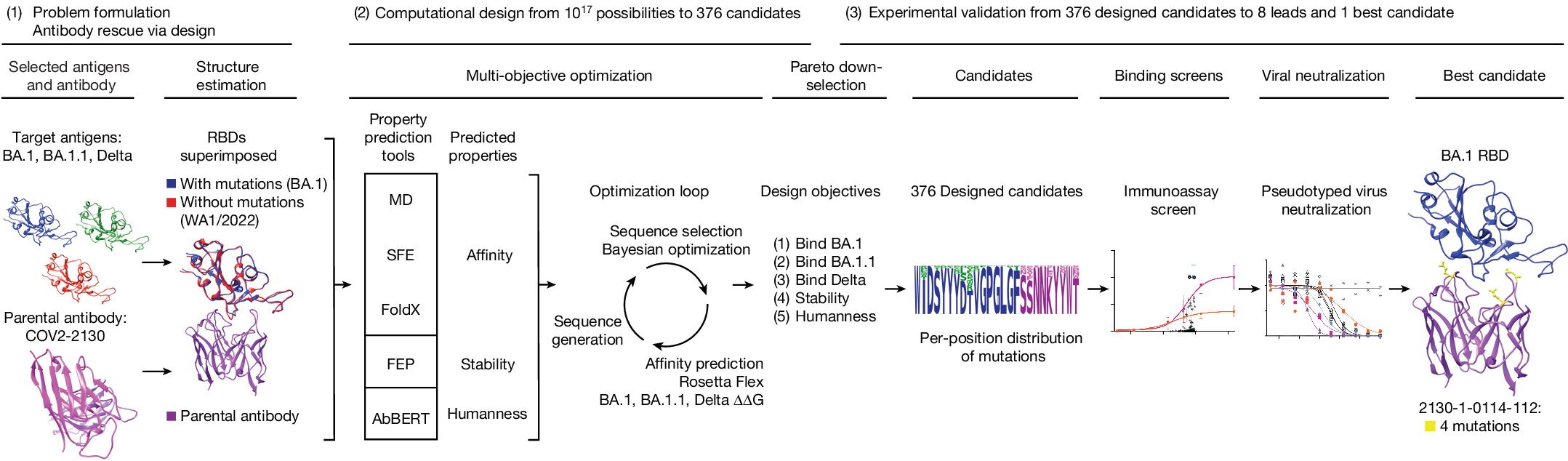
オミクロンに対する臨床抗体の効力を計算で復元する Computationally restoring the potency of a clinical antibody against Omicron
Thomas A. Desautels,Kathryn T. Arrildt,Adam T. Zemla,Edmond Y. Lau,Fangqiang Zhu,Dante Ricci,Stephanie Cronin,Seth J. Zost,Elad Binshtein,Suzanne M. Scheaffer,Bernadeta Dadonaite,Brenden K. Petersen,Taylor B. Engdahl,Elaine Chen,Laura S. Handal,Lynn Hall,John W. Goforth,Denis Vashchenko,Sam Nguyen,Dina R. Weilhammer,Jacky Kai-Yin Lo,Bonnee Rubinfeld,Edwin A. Saada,Tracy Weisenberger,Tri-lab COVID-19 Consortium,… Daniel M. Faissol
Nature Published:08 May 2024
DOI:https://doi.org/10.1038/s41586-024-07385-1
Abstract
The COVID-19 pandemic underscored the promise of monoclonal antibody-based prophylactic and therapeutic drugs1,2,3 and revealed how quickly viral escape can curtail effective options4,5. When the SARS-CoV-2 Omicron variant emerged in 2021, many antibody drug products lost potency, including Evusheld and its constituent, cilgavimab4,5,6. Cilgavimab, like its progenitor COV2-2130, is a class 3 antibody that is compatible with other antibodies in combination4 and is challenging to replace with existing approaches. Rapidly modifying such high-value antibodies to restore efficacy against emerging variants is a compelling mitigation strategy. We sought to redesign and renew the efficacy of COV2-2130 against Omicron BA.1 and BA.1.1 strains while maintaining efficacy against the dominant Delta variant. Here we show that our computationally redesigned antibody, 2130-1-0114-112, achieves this objective, simultaneously increases neutralization potency against Delta and subsequent variants of concern, and provides protection in vivo against the strains tested: WA1/2020, BA.1.1 and BA.5. Deep mutational scanning of tens of thousands of pseudovirus variants reveals that 2130-1-0114-112 improves broad potency without increasing escape liabilities. Our results suggest that computational approaches can optimize an antibody to target multiple escape variants, while simultaneously enriching potency. Our computational approach does not require experimental iterations or pre-existing binding data, thus enabling rapid response strategies to address escape variants or lessen escape vulnerabilities.