2024-07-01 マサチューセッツ工科大学(MIT)
<関連情報>
- https://news.mit.edu/2024/prosthesis-helps-people-with-amputation-walk-naturally-0701
- https://www.nature.com/articles/s41591-024-02994-9
- https://www.science.org/doi/abs/10.1126/scitranslmed.abc5926
バイオニック四肢の連続的神経制御が切断後の生体模倣歩行を回復する Continuous neural control of a bionic limb restores biomimetic gait after amputation
Hyungeun Song,Tsung-Han Hsieh,Seong Ho Yeon,Tony Shu,Michael Nawrot,Christian F. Landis,Gabriel N. Friedman,Erica A. Israel,Samantha Gutierrez-Arango,Matthew J. Carty,Lisa E. Freed & Hugh M. Herr
Nature Medicine Published:01 July 2024
DOI:https://doi.org/10.1038/s41591-024-02994-9
Abstract
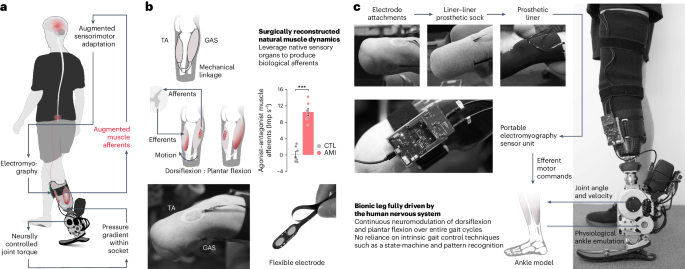
For centuries scientists and technologists have sought artificial leg replacements that fully capture the versatility of their intact biological counterparts. However, biological gait requires coordinated volitional and reflexive motor control by complex afferent and efferent neural interplay, making its neuroprosthetic emulation challenging after limb amputation. Here we hypothesize that continuous neural control of a bionic limb can restore biomimetic gait after below-knee amputation when residual muscle afferents are augmented. To test this hypothesis, we present a neuroprosthetic interface consisting of surgically connected, agonist–antagonist muscles including muscle-sensing electrodes. In a cohort of seven leg amputees, the interface is shown to augment residual muscle afferents by 18% of biologically intact values. Compared with a matched amputee cohort without the afferent augmentation, the maximum neuroprosthetic walking speed is increased by 41%, enabling equivalent peak speeds to persons without leg amputation. Further, this level of afferent augmentation enables biomimetic adaptation to various walking speeds and real-world environments, including slopes, stairs and obstructed pathways. Our results suggest that even a small augmentation of residual muscle afferents restores biomimetic gait under continuous neuromodulation in individuals with leg amputation.
アゴニスト-アンタゴニスト筋神経インターフェース切断により、下肢の固有受容感覚運動神経生理学が維持される Agonist-antagonist myoneural interface amputation preserves proprioceptive sensorimotor neurophysiology in lower limbs
SHRIYA S. SRINIVASAN, GRETA TUCKUTE, JASMINE ZOU, SAMANTHA GUTIERREZ-ARANGO, […], AND HUGH M. HERR
Science Translational Medicine Published:9 Dec 2020
DOI:https://doi.org/10.1126/scitranslmed.abc5926
Preserving proprioception
Limb amputation disrupts sensorimotor signaling, impairing control of neuroprostheses. Srinivasan et al. mapped brain activation via functional magnetic resonance imaging in individuals with agonist-antagonist myoneural interface (AMI) amputation, individuals with traditional lower-limb amputation, and non-amputees. AMI amputation surgically creates autologous muscle-nerve interfaces. The authors found evidence of similar functional activation of the proprioceptive center of the brain, correlated to motor control and muscle activity, in individuals with AMI amputation and non-amputees, whereas individuals with traditional amputation showed reduced activation. Results help illustrate how AMI amputation preserves sensory feedback and motor control.
Abstract
The brain undergoes marked changes in function and functional connectivity after limb amputation. The agonist-antagonist myoneural interface (AMI) amputation is a procedure that restores physiological agonist-antagonist muscle relationships responsible for proprioceptive sensory feedback to enable greater motor control. We compared results from the functional neuroimaging of individuals (n = 29) with AMI amputation, traditional amputation, and no amputation. Individuals with traditional amputation demonstrated a significant decrease in proprioceptive activity, measured by activation of Brodmann area 3a, whereas functional activation in individuals with AMIs was not significantly different from controls with no amputation (P < 0.05). The degree of proprioceptive activity in the brain strongly correlated with fascicle activity in the peripheral muscles and performance on motor tasks (P < 0.05), supporting the mechanistic basis of the AMI procedure. These results suggest that surgical techniques designed to restore proprioceptive peripheral neuromuscular constructs result in desirable central sensorimotor plasticity.