2024-09-05 ミュンヘン大学(LMU)
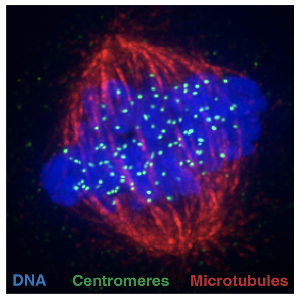
Cell division machinery, made up of microtubule filaments, attaching to centromeres to segregate identical copies of the DNA during cell division. | © Alba Abad Fernandez
<関連情報>
- https://www.lmu.de/en/newsroom/news-overview/news/the-key-to-centromeres-eternal-life-unravelled.html
- https://www.science.org/doi/10.1126/science.ado8270
PLK1を介したリン酸化カスケードがMis18複合体を活性化し、セントロメアの継承を確実にする PLK1-mediated phosphorylation cascade activates Mis18 complex to ensure centromere inheritance
Pragya Parashara, Bethan Medina-Pritchard, Maria Alba Abad, Paula Sotelo-Parrilla, […], and A. Arockia Jeyaprakash
Science Published:5 Sep 2024
DOI:https://doi.org/10.1126/science.ado8270
Editor’s summary
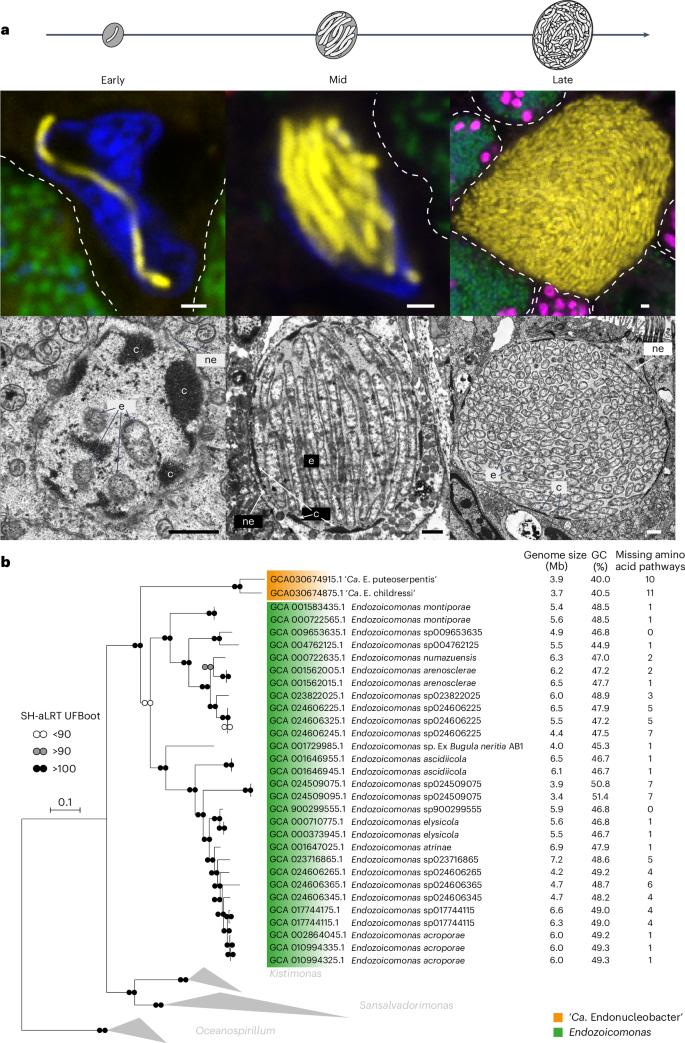
Successful cell division requires precise separation of chromosomes with near perfect fidelity to the daughter cells. Central to this essential process is attachment of the chromosomal centromeres to microtubules of the mitotic spindle. After DNA replication, properly timed regeneration of the centromere and its associated protein complex is essential. Conti et al. and Parashara et al. reveal how Polo-like kinase 1 (PLK-1) coordinates this process at the end of mitosis. PLK-1 has intricate interactions with proteins at the centromere, first phosphorylating associated proteins and then itself binding to those sites, causing conformational changes that promote the recruitment of the histone H3–like protein CENP-A and its chaperone protein HJURP. These results help to explain how the protein complex at the centromere is deposited and regulated to form in a timely manner once each cell cycle. —L. Bryan Ray
Abstract
Accurate chromosome segregation requires the attachment of microtubules to centromeres, epigenetically defined by the enrichment of CENP-A nucleosomes. During DNA replication, CENP-A nucleosomes undergo dilution. To preserve centromere identity, correct amounts of CENP-A must be restored in a cell cycle–controlled manner orchestrated by the Mis18 complex (Mis18α-Mis18β-Mis18BP1). We demonstrate here that PLK1 interacts with the Mis18 complex by recognizing self-primed phosphorylations of Mis18α (Ser54) and Mis18BP1 (Thr78 and Ser93) through its Polo-box domain. Disrupting these phosphorylations perturbed both centromere recruitment of the CENP-A chaperone HJURP and new CENP-A loading. Biochemical and functional analyses showed that phosphorylation of Mis18α and PLK1 binding were required to activate Mis18α-Mis18β and promote Mis18 complex-HJURP interaction. Thus, our study reveals key molecular events underpinning the licensing role of PLK1 in ensuring accurate centromere inheritance.