2024-10-01 ミュンヘン大学(LMU)
<関連情報>
- https://www.lmu.de/en/newsroom/news-overview/news/advance-in-breast-cancer-treatment.html
- https://www.nature.com/articles/s41591-024-03261-7
脳転移の有無にかかわらずHER2陽性進行乳癌に対するトラスツズマブ・デルクステカン:第3b/4相試験 Trastuzumab deruxtecan in HER2-positive advanced breast cancer with or without brain metastases: a phase 3b/4 trial
Nadia Harbeck,Eva Ciruelos,Guy Jerusalem,Volkmar Müller,Naoki Niikura,Giuseppe Viale,Rupert Bartsch,Christian Kurzeder,Michaela J. Higgins,Roisin M. Connolly,Sally Baron-Hay,María Gión,Valentina Guarneri,Giampaolo Bianchini,Hans Wildiers,Santiago Escrivá-de-Romaní,Manoj Prahladan,Helen Bridge,Nataliya Kuptsova-Clarkson,Nana Scotto,Sunil Verma,Nancy U. Lin & the DESTINY-Breast12 study group
Nature Medicine Published:13 September 2024
DOI:https://doi.org/10.1038/s41591-024-03261-7
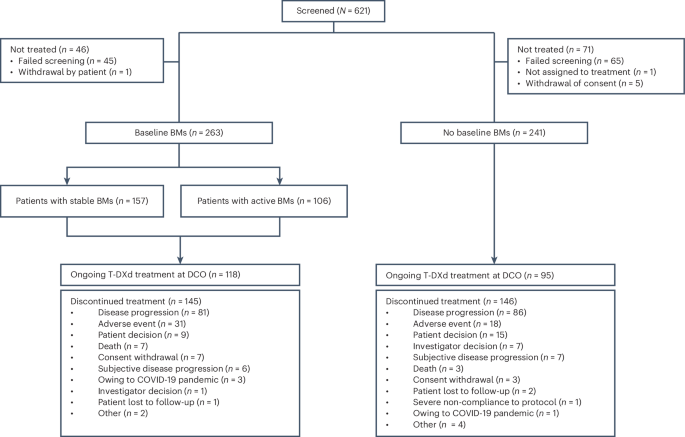
Abstract
Trastuzumab deruxtecan (T-DXd) intracranial activity has been observed in small or retrospective patient cohorts with human epidermal growth factor receptor 2–positive (HER2+) advanced/metastatic breast cancer (mBC) and stable or active (untreated/previously treated and progressing) brain metastases (BMs). The phase 3b/4 DESTINY-Breast12 study investigated T-DXd in patients with HER2+ mBC and is, to our knowledge, the largest prospective study of T-DXd in patients with BMs in this setting. Patients (stable/active BMs (n = 263) and no BMs (n = 241)) treated with one or more prior anti-HER2–based regimens received T-DXd (5.4 mg per kg). Primary endpoints were progression-free survival (PFS; BMs cohort) and objective response rate (ORR) per Response Evaluation Criteria in Solid Tumors version 1.1 (non-BMs cohort). Additional endpoints included central nervous system (CNS) PFS, ORR, time to second progression, CNS ORR (BMs cohort), incidence of new symptomatic CNS metastases (non-BMs cohort), time to progression, duration of response, overall survival and safety (both cohorts). No formal hypothesis testing was conducted for this single-arm, open-label study. In the BMs cohort, 12-month PFS was 61.6% (95% confidence interval (CI): 54.9–67.6), and 12-month CNS PFS was 58.9% (95% CI: 51.9–65.3). In the non-BMs cohort, ORR was 62.7% (95% CI: 56.5–68.8). Grade 3 or higher adverse events occurred in 51% (BMs cohort) and 49% (non-BMs cohort) of patients. Investigator-reported interstitial lung disease/pneumonitis occurred in 16% (grade ≥3: 3%) of patients with BMs and 13% (grade ≥3: 1%) of patients without BMs. These data show substantial and durable overall and intracranial activity for T-DXd, supporting its use in previously treated patients with HER2+ mBC irrespective of stable/active baseline BMs. ClinicalTrials.gov identifier: NCT04739761.


