2024-12-16 ノースウェスタン大学
<関連情報>
- https://news.northwestern.edu/stories/2024/12/what-is-metformins-secret-sauce/
- https://www.science.org/doi/10.1126/sciadv.ads5466
- https://elifesciences.org/articles/02242
メトホルミンはミトコンドリア複合体Iを標的として血糖値を下げる Metformin targets mitochondrial complex I to lower blood glucose levels
Colleen R. Reczek, Ram P. Chakrabarty, Karis B. D’Alessandro, Zachary L. Sebo, […], and Navdeep S. Chandel
Science Advances Published:18 Dec 2024
DOI:https://doi.org/10.1126/sciadv.ads5466

Abstract
Metformin is among the most prescribed antidiabetic drugs, but the primary molecular mechanism by which metformin lowers blood glucose levels is unknown. Previous studies have proposed numerous mechanisms by which acute metformin lowers blood glucose, including the inhibition of mitochondrial complex I of the electron transport chain (ETC). Here, we used transgenic mice that globally express the Saccharomyces cerevisiae internal alternative NADH dehydrogenase (NDI1) protein to determine whether the glucose-lowering effect of acute oral administration of metformin requires inhibition of mitochondrial complex I of the ETC in vivo. NDI1 is a yeast NADH dehydrogenase enzyme that complements the loss of mammalian mitochondrial complex I electron transport function and is insensitive to pharmacologic mitochondrial complex I inhibitors including metformin. We demonstrate that NDI1 expression attenuates metformin’s ability to lower blood glucose levels under standard chow and high-fat diet conditions. Our results indicate that acute oral administration of metformin targets mitochondrial complex I to lower blood glucose.
メトホルミンは癌細胞のミトコンドリア複合体Iを阻害し、腫瘍形成を抑制する Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis
William W Wheaton,Samuel E Weinberg,Robert B Hamanaka,Saul Soberanes,Lucas B Sullivan,Elena Anso,Andrea Glasauer,Eric Dufour,Gokhan M Mutlu,Navdeep S Chandel
eLife Published:May 13, 2014
DOI:https://doi.org/10.7554/eLife.02242
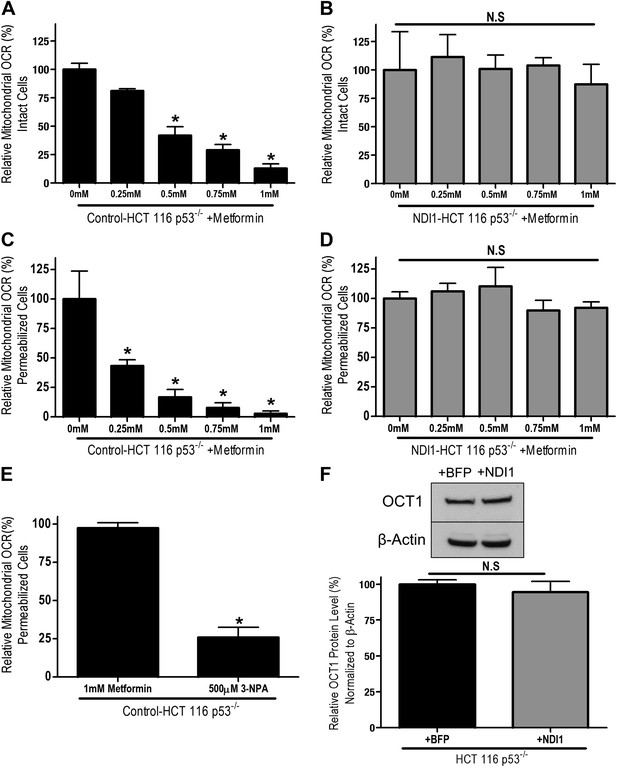
Abstract
Recent epidemiological and laboratory-based studies suggest that the anti-diabetic drug metformin prevents cancer progression. How metformin diminishes tumor growth is not fully understood. In this study, we report that in human cancer cells, metformin inhibits mitochondrial complex I (NADH dehydrogenase) activity and cellular respiration. Metformin inhibited cellular proliferation in the presence of glucose, but induced cell death upon glucose deprivation, indicating that cancer cells rely exclusively on glycolysis for survival in the presence of metformin. Metformin also reduced hypoxic activation of hypoxia-inducible factor 1 (HIF-1). All of these effects of metformin were reversed when the metformin-resistant Saccharomyces cerevisiae NADH dehydrogenase NDI1 was overexpressed. In vivo, the administration of metformin to mice inhibited the growth of control human cancer cells but not those expressing NDI1. Thus, we have demonstrated that metformin’s inhibitory effects on cancer progression are cancer cell autonomous and depend on its ability to inhibit mitochondrial complex I.
- https://doi.org/10.7554/eLife.02242.001
eLife digest
Metformin is widely used to reduce the high blood sugar levels caused by diabetes. Recently, several studies have suggested that patients taking metformin who also develop cancer have tumors that grow more slowly than average. As clinical trials have already started to investigate if metformin is an effective anti-cancer treatment, it is important to understand how it might restrict tumor growth.
Researchers have proposed two ways that metformin could affect tumors. First, insulin is known to prompt cancer cells to divide, so the slower rate of tumor growth could just be a side-effect of the metformin reducing the amount of insulin in the blood. Alternatively, metformin could target cancer cells more directly by cutting the energy supply produced by their mitochondria. Metformin has been shown to disrupt complex I of the electron transport chain that is used by cells to generate energy. However, it is not known if disrupting complex I would actually stop cells dividing because they can generate energy in other ways.
Wheaton, Weinberg et al. have now demonstrated that metformin does target complex I in cancer cells, and that its effects depend on the amount of glucose available for cells to convert, without involving mitochondria, into energy. When there is plenty of glucose, metformin slows down the rate at which cancer cells divide, which slows down tumor growth. When the cells are deprived of glucose, metformin kills the cells instead.
Metformin also inhibits the pathways that regulate hypoxia inducible factors (HIFs), which are part of a system that helps cells to survive low-oxygen conditions, a prominent feature of many tumors. This means that metformin may combat cancer more effectively if used alongside other treatments that reduce the availability of both oxygen and glucose inside cells. Metformin could also potentially treat conditions that are linked to overactive HIFs, such as pulmonary hypertension.
- https://doi.org/10.7554/eLife.02242.002


