2025-03-24 東京科学大学
<関連情報>
アクアグリセロポリンAQP3とGlpFの狭孔構造 Narrowed pore conformations of aquaglyceroporins AQP3 and GlpF
Daisuke Kozai,Masao Inoue,Shota Suzuki,Akiko Kamegawa,Kouki Nishikawa,Hiroshi Suzuki,Toru Ekimoto,Mitsunori Ikeguchi & Yoshinori Fujiyoshi
Nature Communications Published:20 March 2025
DOI:https://doi.org/10.1038/s41467-025-57728-3
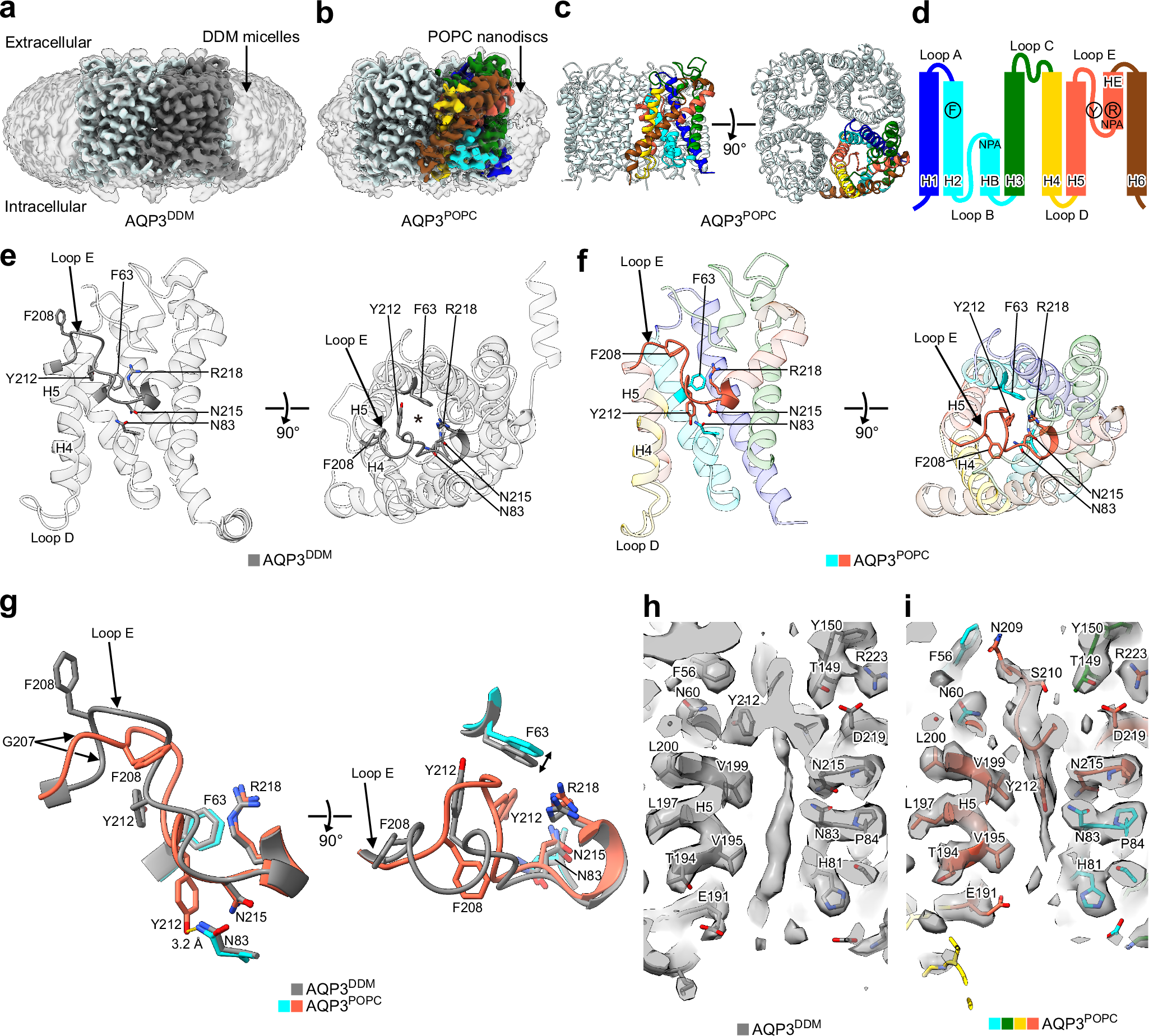
Abstract
Aquaglyceroporins such as aquaporin-3 (AQP3) and its bacterial homologue GlpF facilitate water and glycerol permeation across lipid bilayers. X-ray crystal structures of GlpF showed open pore conformations, and AQP3 has also been predicted to adopt this conformation. Here we present cryo-electron microscopy structures of rat AQP3 and GlpF in different narrowed pore conformations. In n-dodecyl-β-D-maltopyranoside detergent micelles, aromatic/arginine constriction filter residues of AQP3 containing Tyr212 form a 2.8-Å diameter pore, whereas in 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) nanodiscs, Tyr212 inserts into the pore. Molecular dynamics simulation shows the Tyr212-in conformation is stable and largely suppresses water permeability. AQP3 reconstituted in POPC liposomes exhibits water and glycerol permeability, suggesting that the Tyr212-in conformation may be altered during permeation. AQP3 Y212F and Y212T mutant structures suggest that the aromatic residue drives the pore-inserted conformation. The aromatic residue is conserved in AQP7 and GlpF, but neither structure exhibits the AQP3-like conformation in POPC nanodiscs. Unexpectedly, the GlpF pore is covered by an intracellular loop, but the loop is flexible and not primarily related to the GlpF permeability. Our findings illuminate the unique AQP3 conformation and structural diversity of aquaglyceroporins.


