2025-04-30 カロリンスカ研究所(KI)
ChatGPT:
<関連情報>
- https://news.ki.se/new-technology-facilitates-delivery-of-advanced-medicines
- https://www.nature.com/articles/s41467-025-59377-y
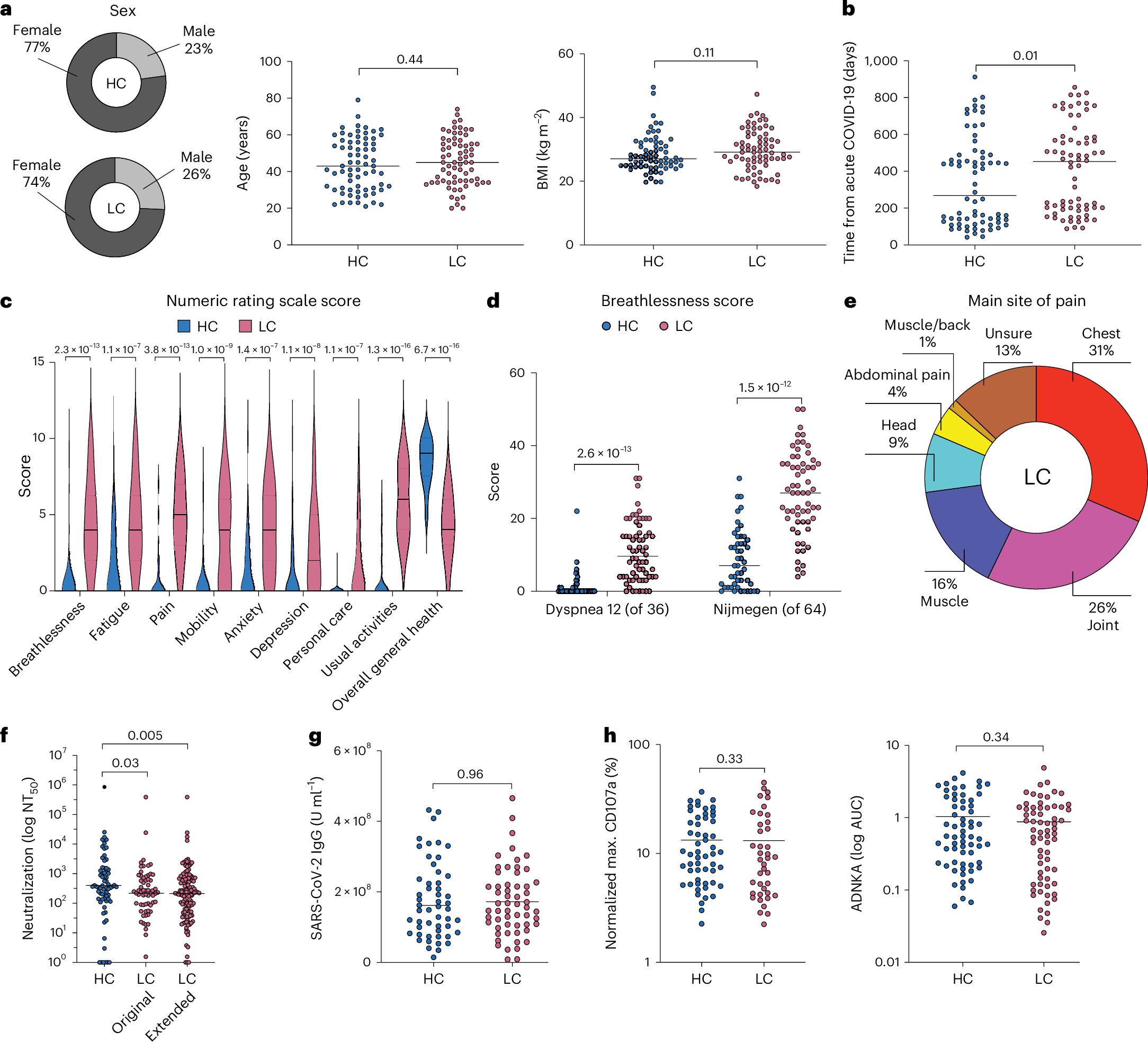
ゲノムエディターを含むマルチモーダル治療薬の効率的細胞内デリバリーのための細胞外小胞のエンジニアリング Engineering of extracellular vesicles for efficient intracellular delivery of multimodal therapeutics including genome editors
Xiuming Liang,Dhanu Gupta,Junhua Xie,Elien Van Wonterghem,Lien Van Hoecke,Justin Hean,Zheyu Niu,Marziyeh Ghaeidamini,Oscar P. B. Wiklander,Wenyi Zheng,Rim Jawad Wiklander,Rui He,Doste R. Mamand,Jeremy Bost,Guannan Zhou,Houze Zhou,Samantha Roudi,H. Yesid Estupiñán,Julia Rädler,Antje M. Zickler,André Görgens,Vicky W. Q. Hou,Radka Slovak,Daniel W. Hagey,… Samir EL Andaloussi
Nature Communications Published:29 April 2025
DOI:https://doi.org/10.1038/s41467-025-59377-y
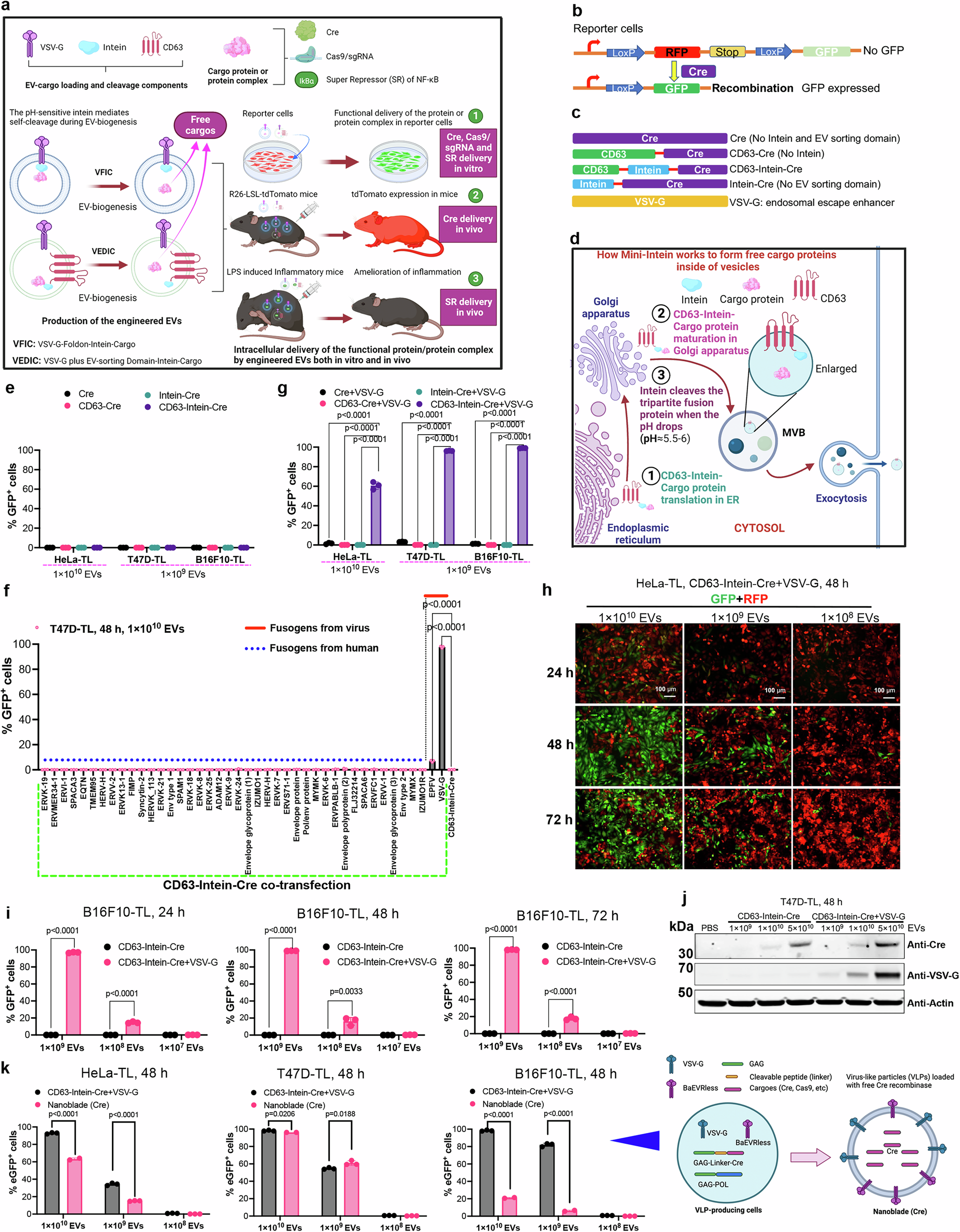
Abstract
Intracellular delivery of protein and RNA therapeutics represents a major challenge. Here, we develop highly potent engineered extracellular vesicles (EVs) by incorporating bio-inspired attributes required for effective delivery. These comprise an engineered mini-intein protein with self-cleavage activity for active cargo loading and release, and fusogenic VSV-G protein for endosomal escape. Combining these components allows high efficiency recombination and genome editing in vitro following EV-mediated delivery of Cre recombinase and Cas9/sgRNA RNP cargoes, respectively. In vivo, infusion of a single dose Cre loaded EVs into the lateral ventricle in brain of Cre-LoxP R26-LSL-tdTomato reporter mice results in greater than 40% and 30% recombined cells in hippocampus and cortex respectively. In addition, we demonstrate therapeutic potential of this platform by showing inhibition of LPS-induced systemic inflammation via delivery of a super-repressor of NF-ĸB activity. Our data establish these engineered EVs as a platform for effective delivery of multimodal therapeutic cargoes, including for efficient genome editing.