2025-05-20 マウントサイナイ医療システム(MSHS)
<関連情報>
- https://www.mountsinai.org/about/newsroom/2025/mount-sinai-researchers-uncover-a-promising-new-way-to-modulate-brain-cell-activity-to-potentially-treat-major-depressive-disorder-in-adults
- https://www.nature.com/articles/s41380-025-02957-7
- https://www.biologicalpsychiatryjournal.com/article/S0006-3223(25)01011-X/abstract
- https://psychiatryonline.org/doi/10.1176/appi.ajp.2020.20050653
うつ病および無気力症患者における腹側被蓋野の活動と結合に対するKCNQカリウムチャネル調節の効果 Effects of KCNQ potassium channel modulation on ventral tegmental area activity and connectivity in individuals with depression and anhedonia
Laurel S. Morris,Sara Costi,Sara Hameed,Katherine A. Collins,Emily R. Stern,Avijit Chowdhury,Carole Morel,Ramiro Salas,Dan V. Iosifescu,Ming-Hu Han,Sanjay J. Mathew & James W. Murrough
Molecular Psychiatry Published:25 March 2025
DOI:https://doi.org/10.1038/s41380-025-02957-7
Abstract
Up to half of individuals with depression do not respond to first-line treatments, possibly due to a lack of treatment interventions informed by neurobiology. A novel therapeutic approach for depression has recently emerged from translational work targeting aberrant activity of ventral tegmental area (VTA) dopamine neurons via modulation of the KCNQ voltage-gated potassium channels. In this study, individuals with major depressive disorder (MDD) with elevated anhedonia were randomized to five weeks of the KCNQ channel opener, ezogabine (up to 900 mg/day) or placebo. Participants completed functional MRI during a monetary anticipation task and resting-state at baseline and at end-of-treatment. The clinical results were reported previously. Here, we examined VTA activity during monetary anticipation and resting-state functional connectivity between the VTA and the ventromedial prefrontal cortex (mesocortical pathway) and ventral striatum (mesolimbic pathway) at baseline and end-of-treatment. Results indicated a significant drug-by-time interaction in VTA activation during anticipation (F(1,34) = 4.36, p = 0.044), where VTA activation was reduced from pre-to-post ezogabine, compared to placebo. Mesocortical functional connectivity was also higher in depressed participants at baseline compared to a healthy control group (t(56) = 2.68, p = 0.01) and associated with VTA hyper-activity during task-based functional MRI at baseline (R = 0.352, p = 0.033). Mesocortical connectivity was also reduced from pre-to-post ezogabine, compared to placebo (significant drug-by-time interaction, F(1,33) = 4.317, p = 0.046). Together this translational work is consistent with preclinical findings highlighting VTA hyper-activity in depression, and suggesting a mechanism of action for KCNQ channel openers in normalizing this hyper-activity in individuals with both depression and anhedonia.
KCNQ(Kv7)チャネル開口薬エゾガビンの大うつ病性障害における線条体脳報酬領域の安静時機能的結合、抑うつ、快感消失に対する効果: ランダム化比較試験の結果 Effects of the KCNQ (Kv7) Channel Opener Ezogabine on Resting-State Functional Connectivity of Striatal Brain Reward Regions, Depression, and Anhedonia in Major Depressive Disorder: Results From a Randomized Controlled Trial
Avijit Chowdhury ∙ Sarah Boukezzi ∙ Sara Costi ∙ … ∙ Sanjay J. Mathew ∙ Laurel Morris ∙ James W. Murrough
Biological Psychiatry Published:March 4, 2025
DOI:https://doi.org/10.1016/j.biopsych.2025.02.897
Abstract
Background
Major depressive disorder (MDD) is a leading cause of disability worldwide, with available treatments often showing limited efficacy. Recent research suggests that targeting specific subtypes of depression and understanding the underlying brain mechanisms can improve treatment outcomes. This study investigates the potential of the potassium KCNQ (Kv7) channel opener ezogabine to modulate the resting-state functional connectivity (RSFC) of the brain’s reward circuitry and alleviate depressive symptoms, including anhedonia, a core feature of MDD.
Methods
A double-blind, randomized, placebo-controlled clinical trial in individuals with MDD ages 18 to 65 years compared daily dosing with ezogabine (n= 19) with placebo (n = 21) for 5 weeks. Functional magnetic resonance imaging assessed RSFC of the brain’s key reward regions (ventral caudate, nucleus accumbens) at baseline and posttreatment. Clinical symptoms were measured using the Snaith-Hamilton Pleasure Scale (SHAPS), Montgomery–Åsberg Depression Rating Scale (MADRS), and other clinical symptom scales.
Results
Ezogabine significantly reduced RSFC between the reward seeds and the posterior cingulate cortex (PCC)/precuneus compared with placebo, which was associated with a reduction in depression severity. Improvements in anhedonia (SHAPS) and depressive symptoms (MADRS) with ezogabine compared with placebo were also associated with decreased connectivity between the reward seeds and mid/posterior cingulate regions (midcingulate cortex, PCC, precuneus).
Conclusions
The findings suggest that ezogabine’s antidepressant effects are mediated through modulation of striatal–mid/posterior cingulate connectivity, indicating a potential therapeutic mechanism for KCNQ-targeted drugs for MDD and anhedonia. Future studies should validate these results in larger trials.
KCNQ2/3チャネル開口薬エゾガビンの報酬回路活性とうつ病の臨床症状への影響: ランダム化比較試験の結果 Impact of the KCNQ2/3 Channel Opener Ezogabine on Reward Circuit Activity and Clinical Symptoms in Depression: Results From a Randomized Controlled Trial
Sara Costi, M.D., Laurel S. Morris, Ph.D., Katherine A. Kirkwood, M.S., Megan Hoch, M.A., Morgan Corniquel, M.A., Brittany Vo-Le, M.S., Tabish Iqbal, M.B.B.S., … , and James W. Murrough, M.D., Ph.D.
The American Journal of Psychiatry Published:3 March 2021
DOI:https://doi.org/10.1176/appi.ajp.2020.20050653
Abstract
Objective:
Preclinical studies point to the KCNQ2/3 potassium channel as a novel target for the treatment of depression and anhedonia, a reduced ability to experience pleasure. The authors conducted the first randomized placebo-controlled trial testing the effect of the KCNQ2/3 positive modulator ezogabine on reward circuit activity and clinical outcomes in patients with depression.
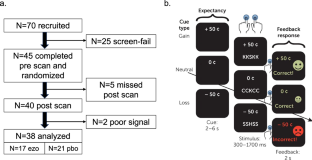
Methods:
Depressed individuals (N=45) with elevated levels of anhedonia were assigned to a 5-week treatment period with ezogabine (900 mg/day; N=21) or placebo (N=24). Participants underwent functional MRI during a reward flanker task at baseline and following treatment. Clinical measures of depression and anhedonia were collected at weekly visits. The primary endpoint was the change from baseline to week 5 in ventral striatum activation during reward anticipation. Secondary endpoints included depression and anhedonia severity as measured using the Montgomery-Åsberg Depression Rating Scale (MADRS) and the Snaith-Hamilton Pleasure Scale (SHAPS), respectively.
Results:
The study did not meet its primary neuroimaging endpoint. Participants in the ezogabine group showed a numerical increase in ventral striatum response to reward anticipation following treatment compared with participants in the placebo group from baseline to week 5. Compared with placebo, ezogabine was associated with a significantly larger improvement in MADRS and SHAPS scores and other clinical endpoints. Ezogabine was well tolerated, and no serious adverse events occurred.
Conclusions:
The study did not meet its primary neuroimaging endpoint, although the effect of treatment was significant on several secondary clinical endpoints. In aggregate, the findings may suggest that future studies of the KCNQ2/3 channel as a novel treatment target for depression and anhedonia are warranted.