2025-05-26 京都府立医科大学
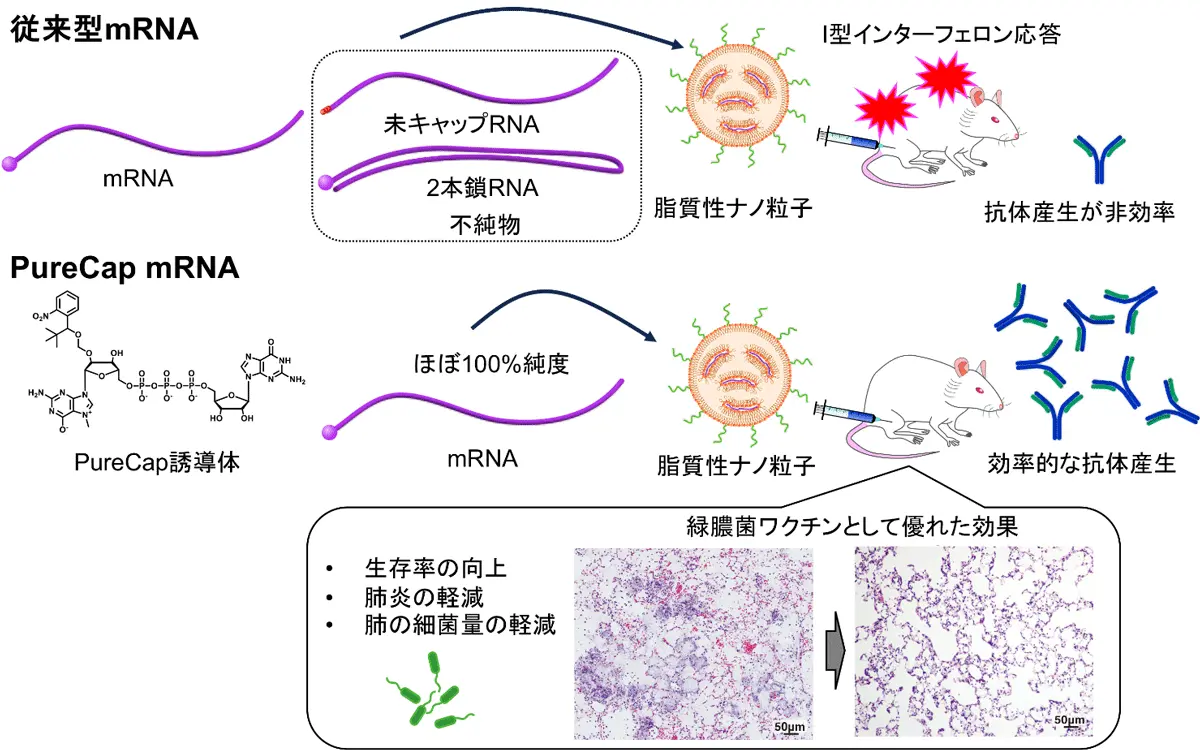
図1. 本研究全体のイメージ図:PureCap技術を用いた緑膿菌mRNAワクチンの開発
<関連情報>
- https://www.kpu-m.ac.jp/doc/news/2025/20250526p.html
- https://www.kpu-m.ac.jp/doc/news/2025/files/38824.pdf
- https://www.sciencedirect.com/science/article/pii/S0168365925004808?via%3Dihub
緑膿菌mRNAワクチンの開発:高純度mRNAワクチンは、I型インターフェロン応答を最小限に抑えることで、緑膿菌に対する強力な免疫効果を発揮する Highly pure mRNA vaccine provides robust immunization against P. aeruginosa by minimizing type I interferon responses
Ken Kawaguchi, Le Bui Thao Nguyen, Mao Kinoshita, Naoko Abe, Makoto Oba, Hiroshi Abe, Kazuki Sudo, Keita Inoue, Satoshi Uchida, Teiji Sawa
Journal of Controlled Release Available online: 16 May 2025
DOI:https://doi.org/10.1016/j.jconrel.2025.113860
Highlights
- Purifying mRNA in mRNA LNP vaccines enhances humoral immune responses.
- Uncapped and double-stranded mRNA impurities lower vaccination efficiency.
- mRNA impurities impair vaccination effects via type I interferon responses.
- Vaccines with highly pure mRNA protect mice from P. aeruginosa infection.
- mRNA vaccines offer an effective approach to combat bacterial infections.
Abstract
Developing effective vaccines against bacteria is critical given the growing threat of antimicrobial resistance (AMR). In this study, we developed mRNA vaccines targeting Pseudomonas aeruginosa (P. aeruginosa), a key AMR pathogen, using PureCap mRNA encapsulated in lipid nanoparticles (LNPs). The PureCap technology offers a facile method for removing immunostimulatory impurities from in vitro transcribed mRNA, such as uncapped RNA and double-stranded RNA (dsRNA). Following intramuscular vaccination of mice with mRNA encoding a model antigen, PureCap mRNA elicited antibody titers that were 26-fold higher than those induced by conventional ARCA-capped mRNA. Mechanistic analyses revealed that both uncapped RNA and dsRNA impurities in ARCA-capped mRNA were responsible for the reduced humoral immune responses. While PureCap mRNA enhanced protein expression efficiency and reduced pro-inflammatory responses compared to ARCA-capped mRNA, minimizing pro-inflammatory responses was particularly critical. When anti-interferon-α/β receptor antibodies were administered, antibody responses to ARCA-capped mRNA vaccination were restored to levels comparable to those achieved with PureCap mRNA vaccination, highlighting the negative impact of type I interferon responses on antibody responses following vaccination with ARCA-capped mRNA. In a vaccination targeting the PcrV protein of P. aeruginosa, PureCap mRNA, but not ARCA-capped mRNA, significantly prolonged the survival of mice following bacterial challenges, presumably due to enhanced antibody production. Furthermore, PureCap mRNA vaccination significantly reduced bacterial loads in the lungs and mitigated tissue damage, edema, and inflammatory responses. These findings underscore the potential of PureCap mRNA as a promising platform for bacterial vaccination, offering a valuable strategy to combat AMR.