2025-05-14 カリフォルニア大学サンフランシスコ校(UCSF)
<関連情報>
- https://www.ucsf.edu/news/2025/05/430001/how-do-middle-aged-folks-get-dementia-it-could-be-these-proteins
- https://www.nature.com/articles/s43587-025-00878-2
脳脊髄液プロテオームの大規模ネットワーク解析により前頭側頭葉変性症の分子シグネチャーが同定される Large-scale network analysis of the cerebrospinal fluid proteome identifies molecular signatures of frontotemporal lobar degeneration
Rowan Saloner,Adam M. Staffaroni,Eric B. Dammer,Erik C. B. Johnson,Emily W. Paolillo,Amy Wise,Hilary W. Heuer,Leah K. Forsberg,Argentina Lario-Lago,Julia D. Webb,Jacob W. Vogel,Alexander F. Santillo,Oskar Hansson,Joel H. Kramer,Bruce L. Miller,Jingyao Li,Joseph Loureiro,Rajeev Sivasankaran,Kathleen A. Worringer,Nicholas T. Seyfried,Jennifer S. Yokoyama,Salvatore Spina,Lea T. Grinberg,William W. Seeley,on behalf of the ALLFTD Consortium,…
Nature Aging Published:16 May 2025
DOI:https://doi.org/10.1038/s43587-025-00878-2
Abstract
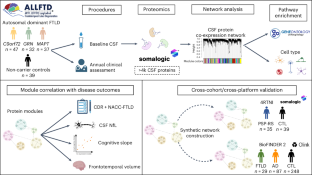
The pathophysiological mechanisms driving disease progression of frontotemporal lobar degeneration (FTLD) and corresponding biomarkers are not fully understood. Here we leveraged aptamer-based proteomics (>4,000 proteins) to identify dysregulated communities of co-expressed cerebrospinal fluid proteins in 116 adults carrying autosomal dominant FTLD mutations (C9orf72, GRN and MAPT) compared with 39 non-carrier controls. Network analysis identified 31 protein co-expression modules. Proteomic signatures of genetic FTLD clinical severity included increased abundance of RNA splicing (particularly in C9orf72 and GRN) and extracellular matrix (particularly in MAPT) modules, as well as decreased abundance of synaptic/neuronal and autophagy modules. The generalizability of genetic FTLD proteomic signatures was tested and confirmed in independent cohorts of (1) sporadic progressive supranuclear palsy-Richardson syndrome and (2) frontotemporal dementia spectrum clinical syndromes. Network-based proteomics hold promise for identifying replicable molecular pathways in adults living with FTLD. ‘Hub’ proteins driving co-expression of affected modules warrant further attention as candidate biomarkers and therapeutic targets.