2025-07-01 京都大学iPS細胞研究所
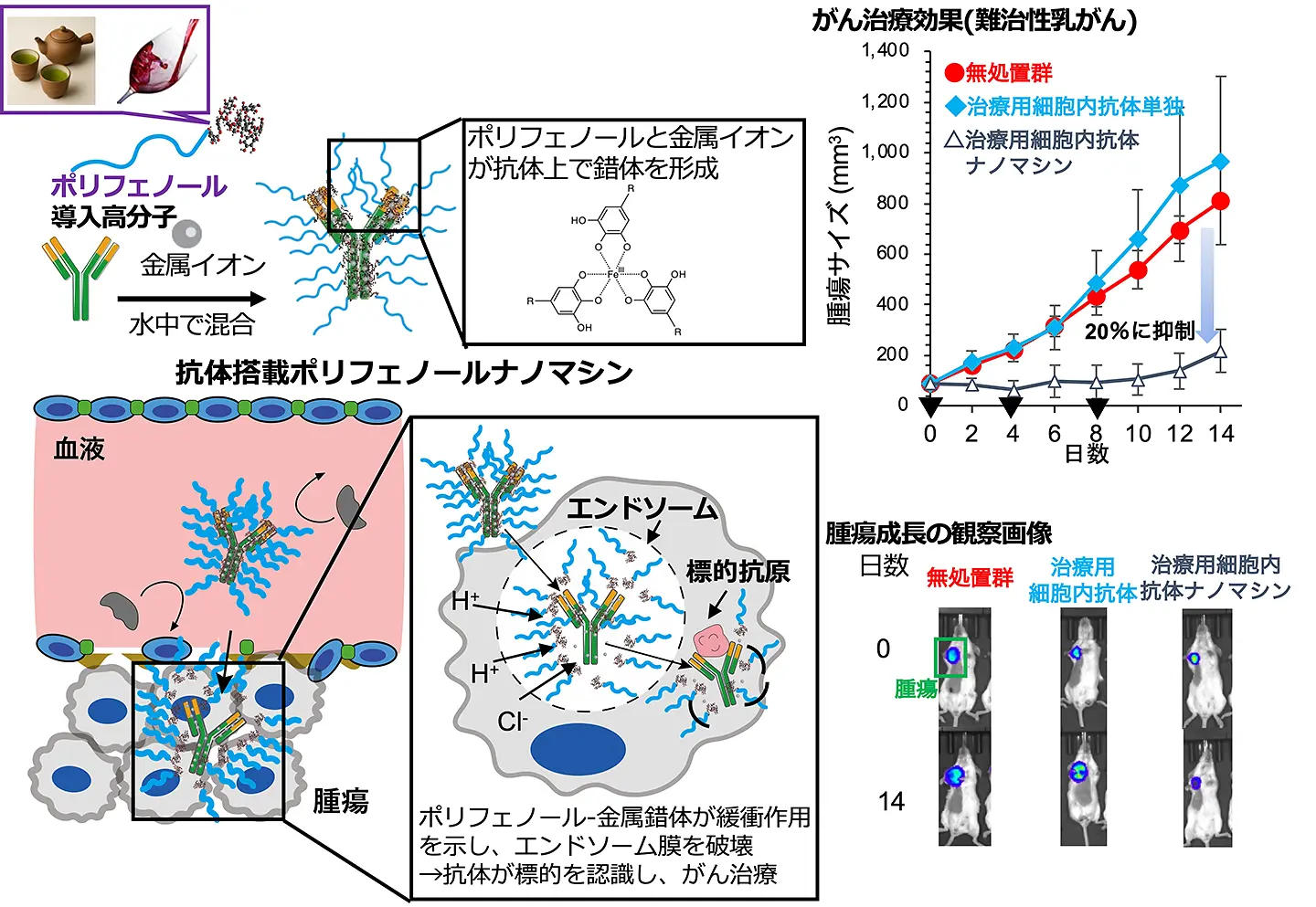
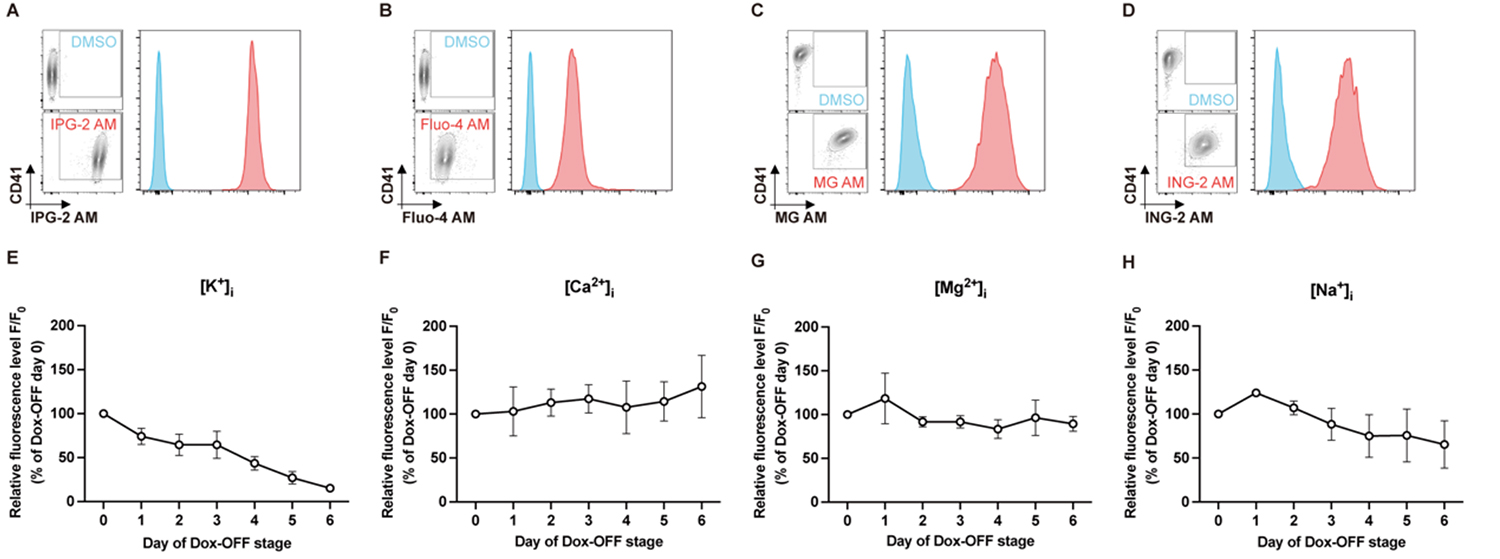 図1:iPS細胞から作製した不死化巨核球細胞株(imMKCL)における、(A) カリウムイオン(K+)、(B) カルシウムイオン(Ca2+)、(C) マグネシウムイオン(Mg2+)、(D) ナトリウムイオン(Na+)の細胞内濃度を示すフローサイトメトリーヒストグラム例。Dox-OFF成熟条件下での培養期間に伴い、細胞内(E) K+濃度が時間依存的に継続して減少していった。一方、(F) Ca2+、(G) Mg2+、(H) Na+の濃度には顕著な変化は認められなかった。
図1:iPS細胞から作製した不死化巨核球細胞株(imMKCL)における、(A) カリウムイオン(K+)、(B) カルシウムイオン(Ca2+)、(C) マグネシウムイオン(Mg2+)、(D) ナトリウムイオン(Na+)の細胞内濃度を示すフローサイトメトリーヒストグラム例。Dox-OFF成熟条件下での培養期間に伴い、細胞内(E) K+濃度が時間依存的に継続して減少していった。一方、(F) Ca2+、(G) Mg2+、(H) Na+の濃度には顕著な変化は認められなかった。
<関連情報>
- https://www.cira.kyoto-u.ac.jp/j/pressrelease/news/250701-100000.html
- https://www.jthjournal.org/article/S1538-7836(25)00331-9/fulltext
KCNN4を介したカリウムイオンの流出は、血小板の生合成につながるミトコンドリア機能を維持する KCNN4-mediated potassium ion efflux maintains mitochondrial functions leading to platelet biogenesis
Qihao Chen ∙ Sou Nakamura ∙ Takuya Yamamoto,, ∙ Naoya Takayama ∙ Naoshi Sugimoto ∙ Koji Eto
Journal of Thrombosis and Haemostasis Published:May 22, 2025
DOI:https://doi.org/10.1016/j.jtha.2025.05.013
Abstract
Background
Potassium ions (K+) are essential for platelet function, yet their role in thrombopoiesis—particularly through specific K+ channels—remains poorly understood. This gap is especially relevant in the context of in vitro platelet production from induced pluripotent stem cell (iPSC)–derived immortalized megakaryocyte progenitor cell lines (imMKCLs), which we developed for clinical-grade platelet manufacturing.
Objectives
We aimed to elucidate how K+ channels contribute to platelet biogenesis, focusing specifically on the calcium ion (Ca2+)-activated K+ channel KCNN4 (also known as KCa3.1).
Methods
Using imMKCLs and human cord blood (CB)–derived megakaryocytes, we analyzed intracellular cation dynamics during platelet production. RNA sequencing profiling was conducted to identify K+ channel gene expression changes, focusing on KCNN4. Its role in proplatelet formation and platelet release was examined using pharmacologic inhibitors and gene knockdown. We further investigated the link between KCNN4 and microtubule organization, mitochondrial function, and reactive oxygen species (ROS) levels.
Results
A progressive decline in intracellular K+ concentration ([K+]ᵢ) was observed during the 6-day maturation period of imMKCLs. KCNN4 was upregulated at onset of platelet generation, and its inhibition or knockdown led to impaired proplatelet formation and reduced platelet yield in both imMKCLs and CB–derived megakaryocytes. These effects were accompanied by decreased [K+]ᵢ, reduced mitochondrial membrane potential (MMP), and increased ROS accumulation.
Conclusion
Our findings reveal that the KCNN4-mediated reduction in [K+]ᵢ is a crucial mechanism linking cytoskeletal reorganization, mitochondrial function, and ROS homeostasis to effective thrombopoiesis. This study provides new insights into platelet biogenesis and offers potential avenues to optimize ex vivo platelet production.