2025-07-15 マウントサイナイ医療システム
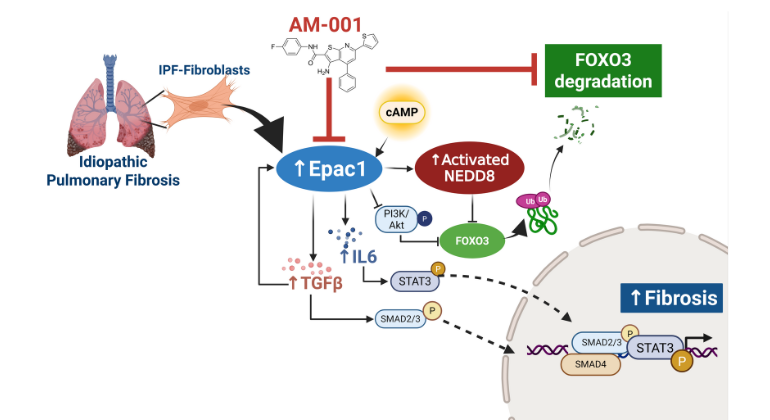
AM-001 blocks a key protein (Epac1), which helps protect another protein (FoxO3a) from being broken down. It also shuts down several harmful signals that drive lung scarring in idiopathic pulmonary fibrosis, helping to reduce lung damage. Image credit: Hadri, et al., European Respiratory Journal
<関連情報>
- https://www.mountsinai.org/about/newsroom/2025/blocking-a-little-known-protein-may-offer-new-hope-for-devastating-lung-disease
- https://publications.ersnet.org/content/erj/early/2025/07/03/1399300302250-2024
Epac1の薬理学的阻害はFoxO3aのNeddyl化を阻害することで肺線維症を予防する Pharmacological Inhibition of Epac1 Protects against Pulmonary Fibrosis by Blocking FoxO3a Neddylation
Katherine Jankowski,Sarah E Lemay,Daniel Lozano-ojalvo,…
European Respiratory Journal Published:10 July 2025
DOI:https://doi.org/10.1183/13993003.02250-2024
Abstract
Background
Idiopathic Pulmonary Fibrosis (IPF) is marked by progressive lung scarring with no existing cure, emphasizing the need for new therapeutic targets. Current evidence suggests that cyclic adenosine monophosphate (cAMP) mitigates lung fibroblast proliferation via the PKA pathway, but the impact of Epac1, a cAMP-activated protein, on IPF remains unexplored.
Objective
To investigate the role of Epac1 in IPF progression.
Methods
We examined lung samples from IPF patients and controls, and from a bleomycin-induced mouse model of pulmonary fibrosis (PF). Epac1’s effects were analysed in knock-out mice and through modulation using viral vectors. The Epac1-specific small compound inhibitor AM-001 was evaluated in vitro using lung fibroblasts from patients with IPF, in vivo in bleomycin mice, and ex vivo in IPF precision cut lung slices.
Results
Increased Epac1 expression was observed in lung tissues from IPF patients, fibrotic fibroblasts, and bleomycin-challenged mice. Genetic or pharmacological inhibition of Epac1 with AM-001 decreased proliferation in normal and IPF fibroblasts, and reduced expression of pro-fibrotic markers such as α-SMA, TGF-β/SMAD2/3, and IL-6/STAT3 pathways. Epac1-specific inhibition consistently protected against bleomycin-induced lung injury and fibrosis, suggesting a significant therapeutic potential. Global gene expression profiling indicated reduced pro-fibrotic gene signature and neddylation pathway components in Epac1-deficient fibroblasts and human-derived lung cells. Mechanistically, the protective effects may involve inhibiting the neddylation pathway and preventing NEDD8 activation, which in turn reduces the degradation of FoxO3a by NEDD8. Additionally, these effects may be enhanced while also limiting the proliferation of lung-infiltrating monocytes.
Conclusions
Our findings demonstrate that Epac1 regulates fibroblast activity in pulmonary fibrosis, and that targeting Epac1 with the pharmacological specific inhibitor AM-001 offers a promising therapeutic approach for treating IPF disease.