2025-08-08 ミュンヘン大学(LMU)
<関連情報>
- https://www.lmu.de/en/newsroom/news-overview/news/resolving-the-structural-basis-of-therapeutic-antibody-function-in-cancer-immunotherapy.html
- https://www.nature.com/articles/s41467-025-61893-w#citeas
RESIを用いたがん免疫療法における治療用抗体の機能の構造的基盤の解明 Resolving the structural basis of therapeutic antibody function in cancer immunotherapy with RESI
Isabelle Pachmayr,Luciano A. Masullo,Susanne C. M. Reinhardt,Jisoo Kwon,Maite Llop,Ondřej Skořepa,Sylvia Herter,Marina Bacac,Christian Klein & Ralf Jungmann
Nature Communications Published:23 July 2025
DOI:https://doi.org/10.1038/s41467-025-61893-w
Abstract
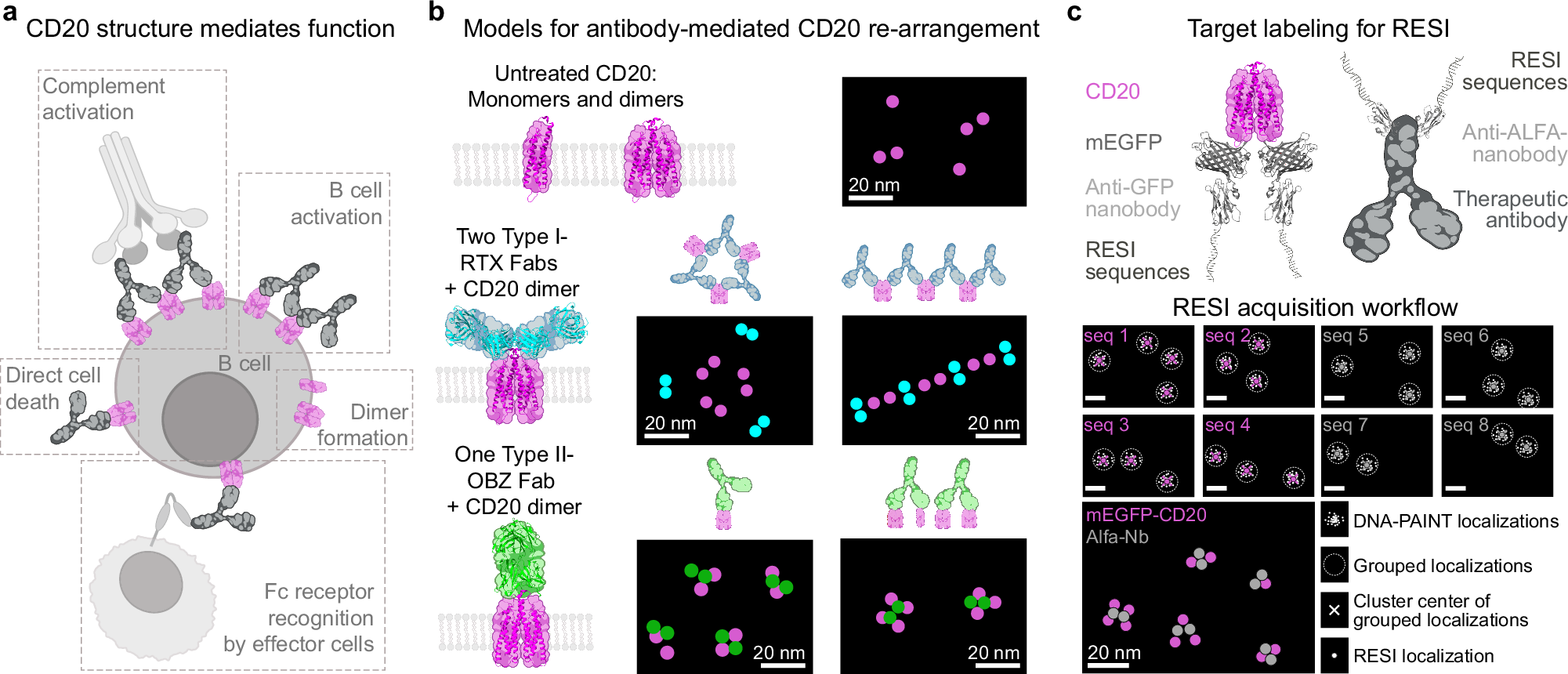
Monoclonal antibodies (mAb) are key therapeutic agents in cancer immunotherapy and exert their effects through Fc receptor-dependent and -independent mechanisms. However, the nanoscale receptor reorganization resulting from mAb binding and its implications for the therapeutic mode of action remain poorly understood. Here, we present a multi-target 3D RESI super-resolution microscopy technique that directly visualizes the structural organization of CD20 receptors and the Type I (e.g., Rituximab) and Type II (e.g., Obinutuzumab) anti-CD20 therapeutic antibodies and quantitatively analyze these interactions at single-protein resolution in situ. We discover that, while Type I mAbs promote higher-order CD20 oligomerization, Type II mAbs induce limited clustering, leading to differences in therapeutic function. Correlating RESI with functional studies for Type II antibodies with different hinge region flexibilities, we show that the oligomeric CD20 arrangement determines the Type I or Type II function. Thus, the nanoscale characterization of CD20-mAb complexes enhances our understanding of the structure-function relationships of therapeutic antibodies and offers insights into the design of next-generation mAb therapies.


