2025-08-21 ペンシルベニア州立大学(PennState)
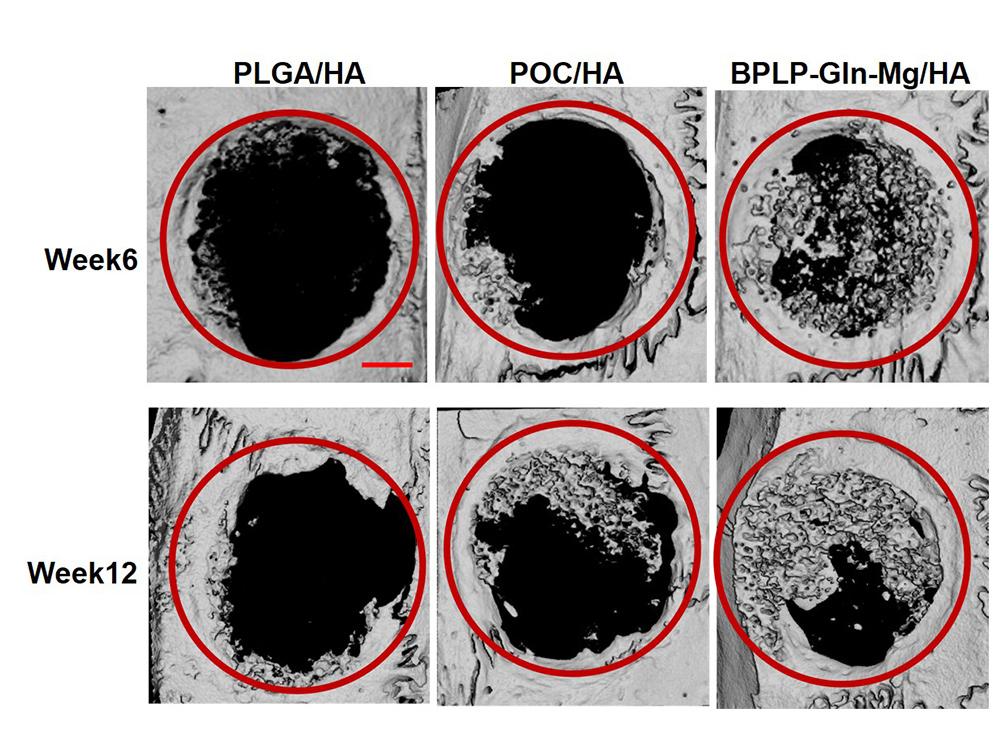
Researchers found that after 12 weeks, the CitraBoneQMg implant, seen in the far right column, had increased the bone growth surrounding rats’ cranial injury by 56% as compared to the rats with the citric acid only-based scaffold (seen in the middle column), and 185% compared to the rats with a traditional bone material implant (seen in the left column). Credit: Provided by Hui Xu/Science Advances. All Rights Reserved.
<関連情報>
- https://www.psu.edu/news/engineering/story/broken-bones-regrow-quickly-help-biodegradable-implant
- https://www.science.org/doi/10.1126/sciadv.ady2862
代謝促進性クエン酸生体材料はクエン酸を介したシグナル伝達経路を介して骨再生を調整するMetabotissugenic citrate biomaterials orchestrate bone regeneration via citrate-mediated signaling pathways
Hui Xu, Xinyu Tan, Ethan Gerhard, Hao Zhang, […] , and Jian Yang
Science Advances Published:23 Jul 2025
DOI:https://doi.org/10.1126/sciadv.ady2862
Abstract
Bone regeneration requires coordinated anabolic and catabolic signaling, yet the interplay between mammalian target of rapamycin complex 1 (mTORC1) and adenosine monophosphate–activated protein kinase (AMPK) pathways remains unclear. This study reveals that citrate, glutamine, and magnesium synergistically activate both pathways via calcium/calmodulin-dependent protein kinase kinase 2 (CaMKK2)– and protein kinase B (Akt)–dependent signaling, bypassing the traditional adenosine monophosphate (AMP)/adenosine triphosphate (ATP) sensing mechanism. This dual activation supports sustained energy metabolism during osteogenesis and challenges the canonical antagonism between mTORC1 and AMPK. We developed CitraBoneQMg, a citrate-based biomaterial incorporating these components via one-pot synthesis. CitraBoneQMg provides sustained release, photoluminescent and photoacoustic imaging capabilities, and tunable mechanical properties. In vitro, it promotes osteogenesis by enhancing alkaline phosphatase (ALP) activity, osteogenic gene expression, and calcium deposition. In vivo, it accelerates bone regeneration in a rat calvarial defect model while promoting anti-inflammatory and neuroregenerative responses. We define this integrated effect as “metabotissugenesis,” offering a metabolically optimized approach to orthopedic biomaterial design.