2025-09-09 マサチューセッツ工科大学(MIT)
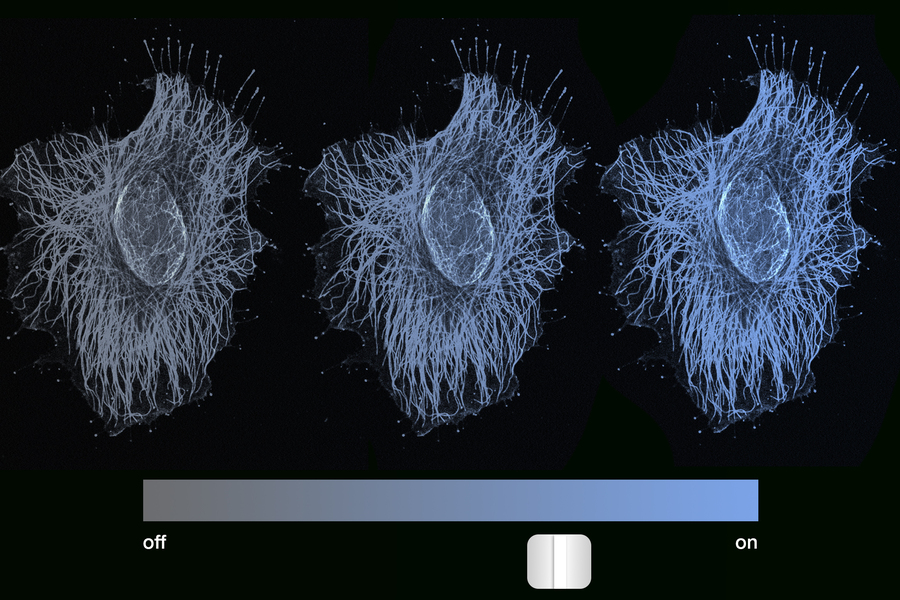
Traditionally, scientists have thought that epigenetic memory locks genes either “on” or “off” — either fully activated or fully repressed. But MIT engineers have found that a cell’s memory is set not only by on/off switching but also through a more graded, dimmer-like dial of gene expression.
Credit: Illustration by Christine Daniloff, MIT; NIH
<関連情報>
- https://news.mit.edu/2025/study-finds-cell-memory-can-be-more-like-dimmer-dial-0909
- https://www.cell.com/cell-genomics/fulltext/S2666-979X(25)00241-1
標的クロマチン編集により明らかになったアナログエピジェネティック記憶 Analog epigenetic memory revealed by targeted chromatin editing
Sebastian Palacios ∙ Simone Bruno ∙ Ron Weiss ∙ … ∙ Andrew Kane ∙ Katherine Ilia, ∙ Domitilla Del Vecchio
Cell Genomics Published:September 9, 2025
DOI:https://doi.org/10.1016/j.xgen.2025.100985
Highlights
- A system was engineered to edit chromatin marks and study gene expression memory
- Gene expression can be memorized at a spectrum of levels, not just “on”’ and “off”
- DNA methylation grade is conserved and mediates gene expression level maintenance
- A model indicates that analog memory emerges in the absence of DNAme-H3K9me3 feedback
Summary
Cells store information by means of chromatin modifications that persist through cell divisions and can hold gene expression silenced over generations. However, how these modifications may maintain other gene expression states has remained unclear. This study shows that chromatin modifications can maintain a wide range of gene expression levels over time, thus uncovering analog epigenetic memory. By engineering a genomic reporter and epigenetic effectors, we tracked the gene expression dynamics following targeted perturbations to the chromatin state. We found that distinct grades of DNA methylation led to corresponding, persistent gene expression levels. Altering the DNA methylation grade, in turn, resulted in permanent loss of gene expression memory. Consistent with experiments, our chromatin modification model indicates that analog memory arises when the positive feedback between DNA methylation and repressive histone modifications is lacking. This discovery will lead to a deeper understanding of epigenetic memory and to new tools for synthetic biology.