2025-10-20 コロンビア大学
<関連情報>
- https://www.cuimc.columbia.edu/news/new-insights-malaria-could-reshape-treatment
- https://www.nature.com/articles/s41467-025-64815-y
抗マラリア標的Pf ATP4の内因性構造は、アピコンプレクサ特異的P型ATPaseモジュレーターを明らかにする Endogenous structure of antimalarial target PfATP4 reveals an apicomplexan-specific P-type ATPase modulator
Meseret T. Haile,Anurag Shukla,James Zhen,Michael W. Mather,Suyash Bhatnagar,Joanne M. Morrisey,Zhening Zhang,Akhil B. Vaidya & Chi-Min Ho
Nature Communications Published:20 October 2025
DOI:https://doi.org/10.1038/s41467-025-64815-y
Abstract
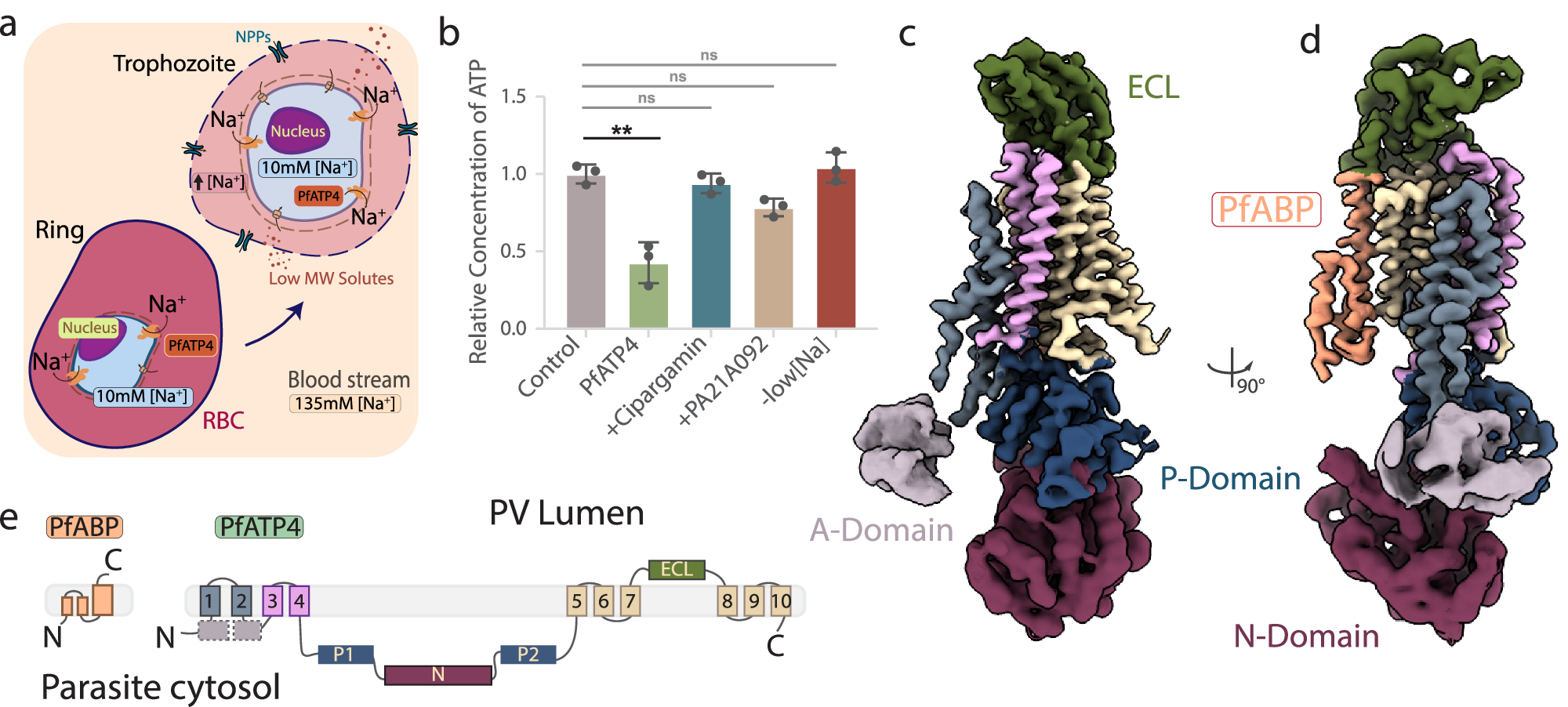
The Plasmodium falciparum sodium efflux pump PfATP4 is a leading antimalarial target, but suffers from a lack of high-resolution structural information needed to identify functionally important features in conserved regions and guide rational design of next generation inhibitors. Here, we determine a 3.7 Å cryoEM structure of PfATP4 purified from CRISPR-engineered P. falciparum parasites, revealing a previously unknown, apicomplexan-specific binding partner, PfABP, which forms a conserved, likely modulatory interaction with PfATP4. The discovery of PfABP presents an unexplored avenue for designing PfATP4 inhibitors.