2025-10-24 東京農工大学
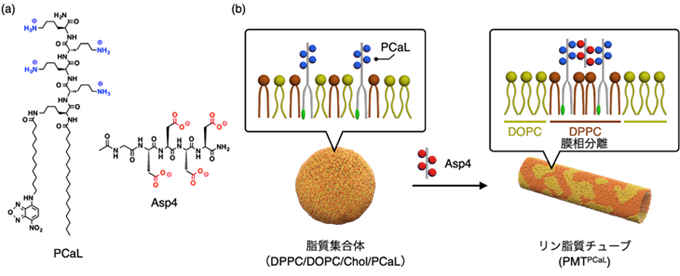
図1 (a) PCaLとAsp4の分子構造、(b) PCaLの集合により引き起こされる膜相分離とリン脂質マイクロチューブ(PMTPCaL)の形成の概略図。
DPPC/DOPC/Chol/PCaLからなる四成分膜にAsp4を添加すると、PCaLの集合化と共に膜相分離が誘導され、PMTPCaLが形成される。
<関連情報>
- https://www.tuat.ac.jp/outline/disclosure/pressrelease/2025/20251024_02.html
- https://www.tuat.ac.jp/documents/tuat/outline/disclosure/pressrelease/2025/20251024_02.pdf
- https://pubs.acs.org/doi/10.1021/jacs.5c13384
膜相分離法によって構築された構造的に強固なリン脂質マイクロチューブは、膜結合タンパク質のチューブ内特性評価のための足場として機能します A Structurally Robust Phospholipid Microtube Constructed by Membrane Phase Separation as a Scaffold for On-Tube Characterization of Membrane-Bound Proteins
Noriyuki Uchida,Ryu Ishizaka,Anju Kawakita,Hiroshi Ueno,Hiroyuki Noji,Rinshi S. Kasai,Takeshi Yokoyama,Saburo Kurihara,Tomoki Noguchi,Go Watanabe,Ayaka Iwasaki,Itsuki Ajioka,Kazuyoshi Muranishi,Ken Yoshizawa,Shingo Kanemura,Masaki Okumura,and Takahiro Muraoka
Journal of the American Chemical Society Published: October 23, 2025
DOI:https://doi.org/10.1021/jacs.5c13384
Abstract
Artificial phospholipid assemblies, such as liposomes, have become indispensable scaffolds for the characterization of membrane proteins. Phospholipid microtubes (PMTs) are universal biological architectures, as seen in the endoplasmic reticulum and neurites, that are constructed by curvature-sensing membrane-bound proteins such as Bin/Amphiphysin/Rvs (BAR) proteins. Inspired by the biological PMTs, artificial PMTs have been constructed by physically pulling the membranes using optical tweezers or kinesin motors. However, the inherent low stability of artificial PMTs, which collapse after the removal of the energy source, has critically limited their applications as scaffolds. Here, we report the construction of structurally robust PMTs as practically useful scaffolds for on-tube characterization of membrane-bound proteins. We focused on a membrane deformation driven by phase separation between saturated and unsaturated phospholipids. We developed a polycationic peptide lipid (PCaL) that dissociates the phase separation. Interestingly, complexation of PCaL with an anionic ligand prompted the spontaneous formation of phospholipid microtubes (PMTPCaL). Importantly, PMTPCaL exhibited high robustness against harsh physical stresses, including increased temperatures, increased salt concentrations, osmotic stress, physical pulling using optical tweezers, and molecular crowding. Taking advantage of the high structural stability, PMTPCaL was utilized as a scaffold for on-tube characterization of a curvature-sensing membrane-bound protein. We revealed that sorting nexin-1 enhances its binding property with a tubular membrane under highly crowded cell-mimicking conditions relative to noncrowding conditions.