2025-11-03 ワシントン大学セントルイス校
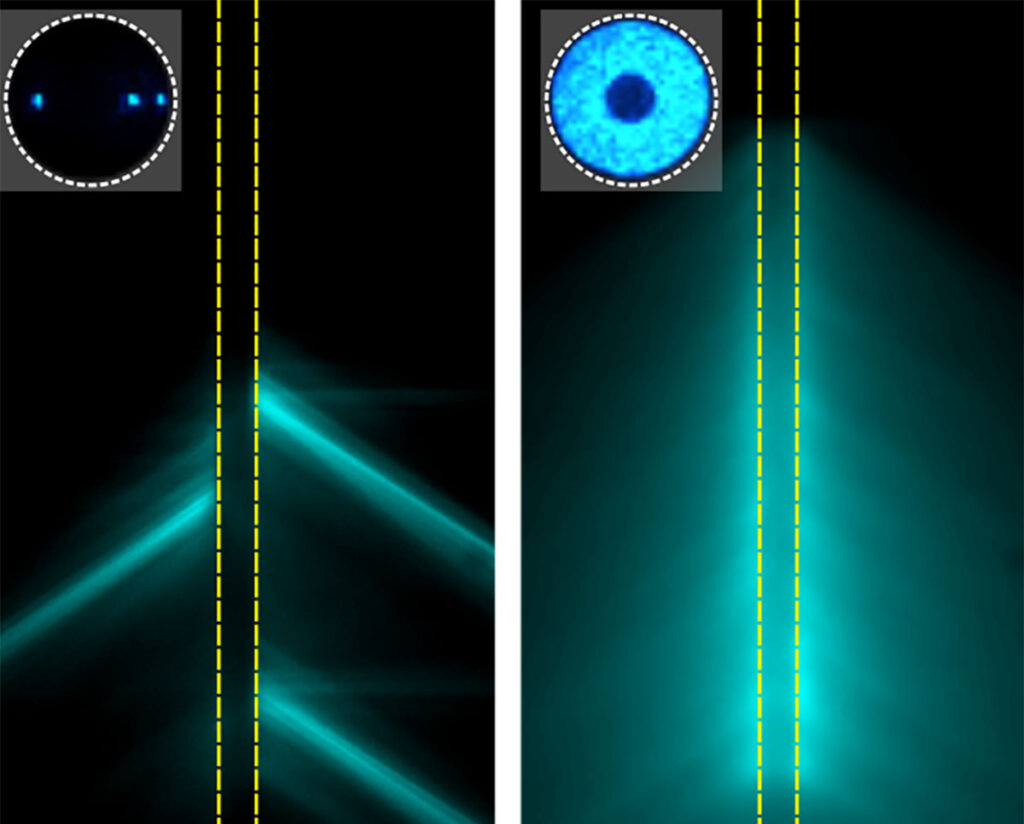
WashU researchers have discovered why cells communicate mechanically through the extracellular matrix, but only within a critical distance of approximately 100-200 micrometers. (Image: Wenyu Kong/Tsinghua University)
<関連情報>
- https://source.washu.edu/2025/11/tissue-tipping-points-how-cells-collectively-switch-from-healthy-to-disease-states/
- https://engineering.washu.edu/news/2025/Tissue-tipping-points-How-cells-collectively-switch-from-healthy-to-disease-states.html
- https://www.pnas.org/doi/10.1073/pnas.2514995122
繊維リクルートメントは、マトリックス介在型組織リモデリングにおける臨界細胞間隔での細胞分極の相転移を促進する Fiber recruitment drives a phase transition of cell polarization at a critical cell spacing in matrix-mediated tissue remodeling
Xiangjun Peng, Yuxuan Huang, Wenyu Kong, +3 , and Guy M. Genin
Proceedings of the National Academy of Sciences Published:October 3, 2025
DOI:https://doi.org/10.1073/pnas.2514995122
Significance
This study reveals how cell-to-cell mechanotransduction through extracellular matrix (ECM) determines outcomes in a wide range of physiological and pathological processes. Using bio-chemo-mechanical methods, we dissect how cell spacing and shape interact with nonlinear ECM mechanics to determine how cells influence the contractility and polarization of their neighbors. This provides a mechanistic explanation for the concepts of optimal ECM rigidity and paratensile signaling. In addition to filling a critical gap in our understanding of cellular interactions in fibrosis and wound healing, results offer insights into how cell spreading facilitates long-range force transmission within tissues in disease and health, and offer insight into how manipulating the physical microenvironment may enable more effective treatments for fibrosis, cancer progression, and chronic wounds.
Abstract
Biological tissues exhibit sharp phase transitions where cells collectively transition from disordered to ordered states at critical densities. We demonstrate through bio-chemo-mechanical modeling that this emergent behavior arises from a nonmonotonic dependence on nonlinear extracellular matrix (ECM) mechanics: mechanical communication between cells is optimized at intermediate stiffness values where cells can both generate sufficient forces and create strain-stiffened tension bands in the ECM. This balance establishes a critical cell spacing threshold for cell–cell communication (100 to 200 μm) that is conserved across experimental observations for a broad range of cell types and collagen densities. Our model reveals that the critical stretch ratio at which fibrous networks transition from compliant to strain-stiffening governs this threshold through the formation of tension bands between neighboring cells. These mechanical communication networks drive collective phase transition in tissue condensation when cell density exceeds an effective percolation threshold. Our model explains how microscale cell–ECM interactions control emergent mechanical properties in biological systems and offers insight both into the physics of inhomogeneous materials under active stress, and into potential mechanical interventions for wound healing and fibrotic disorders.