2025-11-20 イェール大学
<関連情報>
- https://medicine.yale.edu/news-article/reversing-fibrosis-new-research-provides-insight-for-novel-therapies/
- https://www.nature.com/articles/s41467-025-64648-9
- https://ashpublications.org/blood/article/doi/10.1182/blood.2025029836/547298/Sclerotic-GVHD-and-Scleroderma-Share-Dysregulated
- https://www.science.org/doi/10.1126/sciimmunol.abq6691
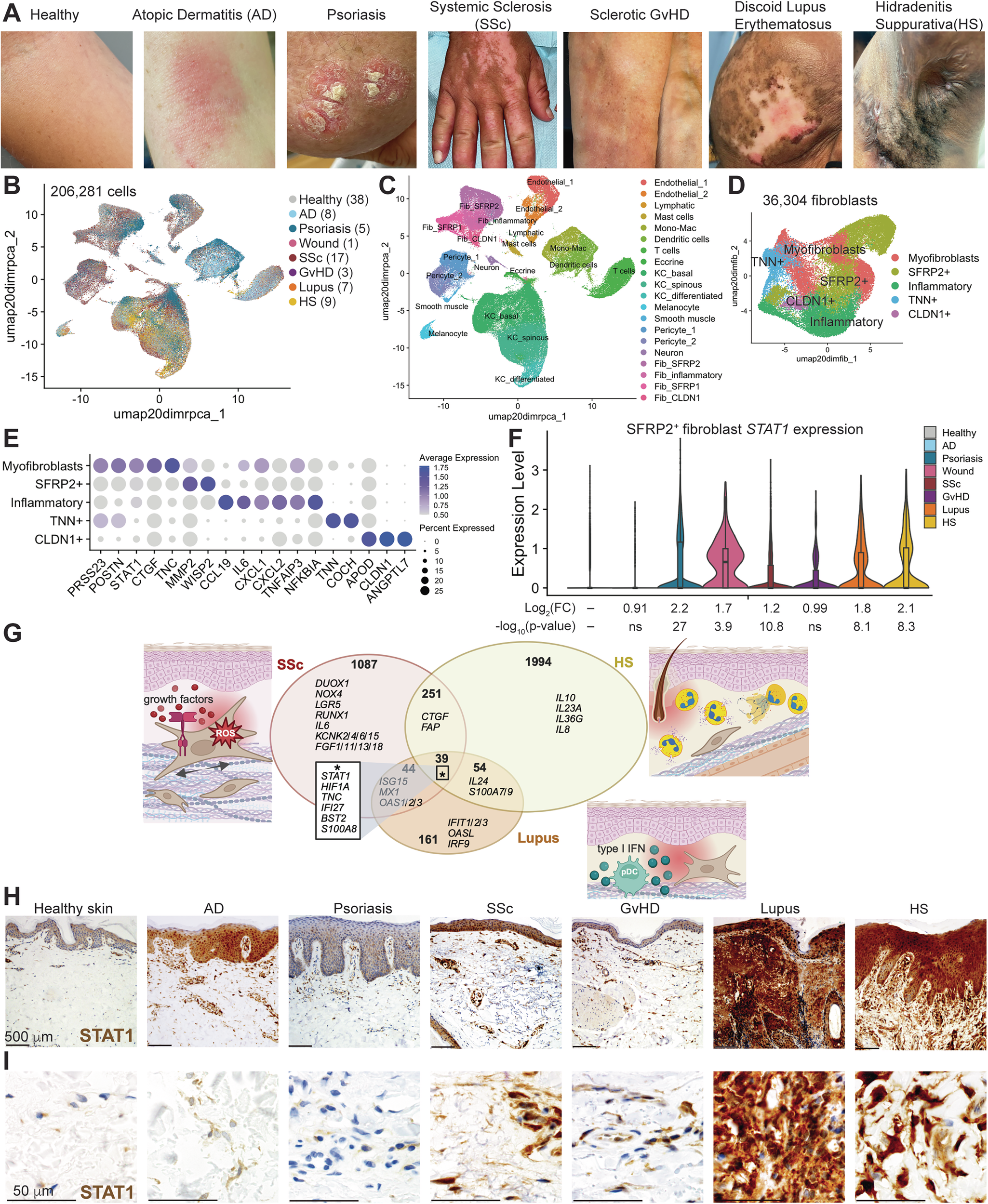
EGFR-STAT1経路は線維炎症性皮膚疾患における線維化の開始を促進する EGFR-STAT1 pathway drives fibrosis initiation in fibroinflammatory skin diseases
Anahi V. Odell,Nathan M. Newton,Anna Eisenstein,Goran Micevic,Richard A. Flavell & Ian D. Odell
Nature Communications Published:09 October 2025
DOI:https://doi.org/10.1038/s41467-025-64648-9
Abstract
Chronic inflammatory skin diseases affect the health of millions of people worldwide, and those that feature fibrosis are refractory to treatments. The signals that determine whether fibrosis occurs during chronic skin inflammation are poorly understood. We generated a scRNA-seq atlas of seven inflammatory skin diseases and their healthy controls. Diseases with fibrosis demonstrate higher expression and activity of STAT1 in fibroblasts. Fibroblast STAT1 is required for skin fibrosis development in mice. STAT1 activation promotes a fibrotic gene expression profile which can be activated directly by EGFR in a JAK-independent manner and abrogated by genetic and pharmacologic STAT1 inhibition. The EGFR-STAT1 pathway is stimulated by high affinity EGFR ligands expressed by activated keratinocytes, suggesting keratinocyte-derived signals as triggers of skin fibrosis. In sum, fibroinflammatory skin diseases are characterized by fibroblast EGFR-STAT1 signaling that controls expression of fibrotic genes, elucidating an interferon independent function of STAT1 to mediate fibrotic skin diseases.
硬化性GVHDと強皮症は遺伝子発現の異常を共有しており、EREG治療抗体によって改善されるSclerotic GVHD and Scleroderma Share Dysregulated Gene Expression that is Ameliorated by EREG Therapeutic Antibody
Nathan M Newton,Kriti Agrawal,Anahi V Odell,Timothy Scott Tracy,Craig Stanway Hackett,Anne B. Eldrup,Michael Whitfield,Viktor Martyanov,Michael Girardi,Esen Sefik,Richard A. Flavell,Ian D Odell
Blood Published:September 17, 2025
DOI:https://doi.org/10.1182/blood.2025029836
Key Points
- New fully human anti-EREG therapeutic antibody is highly developable and demonstrates antifibrotic capabilities in vivo.
- Sclerotic GvHD and SSc share EREG-TNC-TLR4 signaling axis that is reduced by anti-EREG treatment in patient skin explants.
Immune driven fibrotic skin diseases including scleroderma/systemic sclerosis (SSc) and chronic graft-versus-host-disease (cGvHD) cause skin stiffening that has major impact on patient quality of life and associated patient mortality. Therapies to improve sclerotic skin resulting from these diseases are largely ineffective. We previously showed that EREG, a DC3 dendritic cell-derived EGFR ligand, is elevated in the skin and lung of patients with SSc and required for maintenance of skin fibrosis. Here, we developed a fully human anti-EREG neutralizing antibody that has both high affinity and specificity. We found this therapeutic antibody to be functional and safe in vivo using human EREG knock-in mice. To understand the antifibrotic mechanism of targeting EREG, we aligned skin single-cell transcriptomic profiles of SSc, morphea (localized scleroderma) and SclGvHD with disease biomarkers. EREG expression in the skin was elevated in all three fibrotic diseases and a driver of TNC production by myofibroblasts in all three fibrotic diseases. TNC is a pro-inflammatory extracellular glycoprotein that functions as an endogenous TLR4 ligand which induces expression of TLR4 target genes CCL2 and IL6. Examination of skin explants from patients with active SclGvHD treated with anti-EREG therapeutic antibody by spatial transcriptomics demonstrated upregulation of matrix degradation by increased MMP and decreased TIMP1 expression. Protein measurements showed reduced secretion of EREG targets TNC, CCL2, and TIMP1 in all patients, and type I collagen and FN1 in 3/4 patients. Thus, sclerotic skin treated with the anti-EREG therapeutic antibody reduced inflammatory and fibrosis biomarkers associated with EGFR and TLR4 signaling.
エピレグリンは、皮膚と肺の線維化を維持する樹状細胞由来のEGFRリガンドである Epiregulin is a dendritic cell–derived EGFR ligand that maintains skin and lung fibrosis
Ian D. Odell, Holly Steach, Stephen B. Gauld, Lauren Reinke-Breen, […] , and Richard A. Flavell
Science Immunology Published:9 Dec 2022
DOI:https://doi.org/10.1126/sciimmunol.abq6691
Hitting the brakes on fibrosis
While tissue fibrosis is the culminating event of many human inflammatory diseases, few anti-fibrotic therapies are available and the cellular and molecular mechanisms driving fibrosis remain unclear. Using single cell transcriptomics, Odell et al. found that skin from patients with diffuse cutaneous systemic sclerosis was enriched for dendritic cells (DCs) producing the epidermal growth factor receptor (EGFR) ligand epiregulin. DC production of epiregulin could be induced by type I interferon and promoted NOTCH-mediated extracellular matrix gene expression in fibroblasts. In mouse models of bleomycin-induced skin and lung fibrosis, an epiregulin-neutralizing antibody alleviated fibrosis. These results identify a role for epiregulin-producing DCs in maintaining fibrosis and suggest that blocking epiregulin’s EGFR activity could be a promising therapeutic strategy for treating fibrotic diseases.
Abstract
Immune cells are fundamental regulators of extracellular matrix (ECM) production by fibroblasts and have important roles in determining extent of fibrosis in response to inflammation. Although much is known about fibroblast signaling in fibrosis, the molecular signals between immune cells and fibroblasts that drive its persistence are poorly understood. We therefore analyzed skin and lung samples of patients with diffuse cutaneous systemic sclerosis, an autoimmune disease that causes debilitating fibrosis of the skin and internal organs. Here, we define a critical role of epiregulin-EGFR signaling between dendritic cells and fibroblasts to maintain elevated ECM production and accumulation in fibrotic tissue. We found that epiregulin expression marks an inducible state of DC3 dendritic cells triggered by type I interferon and that DC3-derived epiregulin activates EGFR on fibroblasts, driving a positive feedback loop through NOTCH signaling. In mouse models of skin and lung fibrosis, epiregulin was essential for persistence of fibrosis in both tissues, which could be abrogated by epiregulin genetic deficiency or a neutralizing antibody. Therapeutic administration of epiregulin antibody reversed fibrosis in patient skin and lung explants, identifying it as a previously unexplored biologic drug target. Our findings reveal epiregulin as a crucial immune signal that maintains skin and lung fibrosis in multiple diseases and represents a promising antifibrotic target.