2025-11-24 ワシントン大学セントルイス校
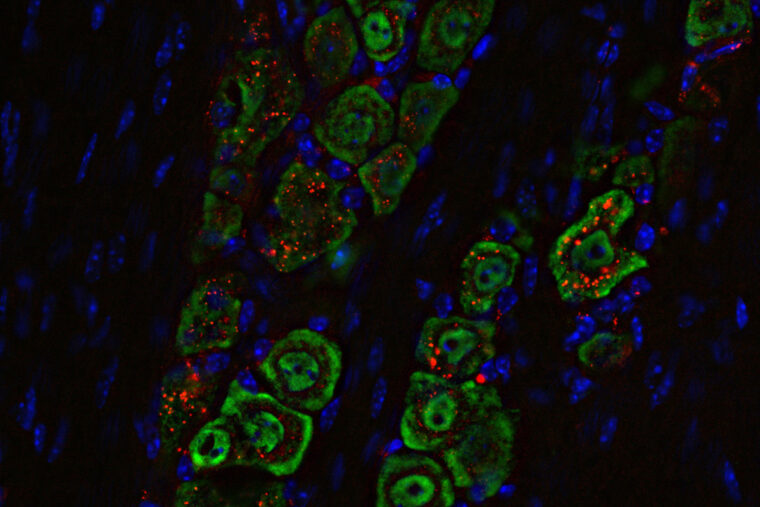
Researchers at WashU Medicine have developed a noninvasive medicine delivered through the nose that successfully eliminated deadly brain tumors in mice. The medicine is based on a spherical nucleic acid, a nanomaterial (labeled red) that travels along a nerve (green) from the nose to the brain, where it triggers an immune response to eliminate the tumor. (Image courtesy of Alexander Stegh)
<関連情報>
- https://source.washu.edu/2025/11/nasal-drops-fight-brain-tumors-noninvasively/
- https://medicine.washu.edu/news/nasal-drops-fight-brain-tumors-noninvasively/
- https://www.pnas.org/doi/10.1073/pnas.2409557122
cGAS作動性球状核酸は神経膠芽腫の免疫微小環境を再プログラムし、抗腫瘍免疫を促進する cGAS-agonistic spherical nucleic acids reprogram the glioblastoma immune microenvironment and promote antitumor immunity
Akanksha S. Mahajan, Corey Dussold, Seunghyun Kim, +9 , and Alexander H. Stegh
Proceedings of the National Academy of Sciences Published:November 3, 2025
DOI:https://doi.org/10.1073/pnas.2409557122
Significance
Intratumorally delivered STING-agonistic cyclic dinucleotides (CDN) exhibit therapeutic benefit in preclinical glioblastoma (GBM) models, but inadequate bioavailability limits CDN therapy to invasive intratumoral administration. Here, we have designed, synthesized, and developed a first-in-class cGAS- agonistic spherical nucleic acid (SNA) structure that can reprogram the immunosuppressive GBM immune microenvironment by triggering cGAS–STING pathway activation. When administered via noninvasive nose-to-brain delivery, this strategy elicits potent antiglioma activity and overcomes immune checkpoint inhibitor treatment resistance. The use of SNAs addresses the challenges of noninvasive nucleic acid delivery to intracranial tumor sites and establishes ISD45-SNAs as a immunotherapeutic modality for GBM treatment.
Abstract
The cyclic GMP-AMP synthase-Stimulator of Interferon Genes (cGAS–STING) pathway is an important DNA-sensing mechanism that increases T cell trafficking and activation in tumors and reverses the immunosuppressive phenotype of myeloid cells. Therefore, direct STING targeting using synthetic cyclic dinucleotides (CDNs) is an attractive strategy for treating lymphocyte-depleted and myeloid cell–enriched tumors, such as glioblastoma (GBM). However, inadequate bioavailability and poor cellular accumulation limit the clinical development of CDNs, particularly for noninvasive administration strategies. Spherical nucleic acids (SNAs) have emerged as promising modular constructs for creating therapeutic lead compounds for many diseases, including different forms of cancer. Here, we report the development of cGAS-activating SNAs that consist of gold nanoparticle cores functionalized with a shell of densely packed interferon-stimulatory DNA oligonucleotides (ISD45-SNAs). These nanostructures bind to cGAS, the sensor of cytosolic dsDNA upstream of STING, promoting the catalytic production of endogenous CDNs and downstream STING activation more potently than clinically tested CDNs. When administered intranasally or intratumorally to poorly immunogenic syngeneic GBM mouse models, ISD45-SNAs inhibit tumor growth more effectively than CDNs and promote long-term animal subject survival through specific cGAS–STING pathway activation. ISD45-SNAs induce a proinflammatory immune microenvironment enriched with effector T cells and proinflammatory macrophages. When coadministered with immune checkpoint inhibitors (ICI), they abolish GBM tumor development and induce long-term antiglioma immunity. These studies establish ISD45-SNAs as an immune-stimulatory modality for triggering innate and adaptive immune responses and increasing ICI efficacy for GBM treatment.