2025-12-02 ロックフェラー大学
<関連情報>
- https://www.rockefeller.edu/news/38697-new-model-demonstrates-how-a-dynamic-mechanism-regulates-traffic-through-the-nuclear-pore-complex/
- https://www.nature.com/articles/s41556-025-01812-9
カリオフェリンは核膜孔複合体輸送バリアの動的組織を再構築する Karyopherins remodel the dynamic organization of the nuclear pore complex transport barrier
Toshiya Kozai,Javier Fernandez-Martinez,Larisa E. Kapinos,Paola Gallardo,Trevor van Eeuwen,Martin Saladin,Roi Eliasian,Adam Mazur,Wenzhu Zhang,Jeremy Tempkin,Radhakrishnan Panatala,Maria Delgado-Izquierdo,Raul Escribano-Marin,Qingzhou Feng,Chenxiang Lin,Andrej Sali,Brian T. Chait,Barak Raveh,Liesbeth M. Veenhoff,Michael P. Rout & Roderick Y. H. Lim
Nature Cell Biology Published:02 December 2025
DOI:https://doi.org/10.1038/s41556-025-01812-9
Abstract
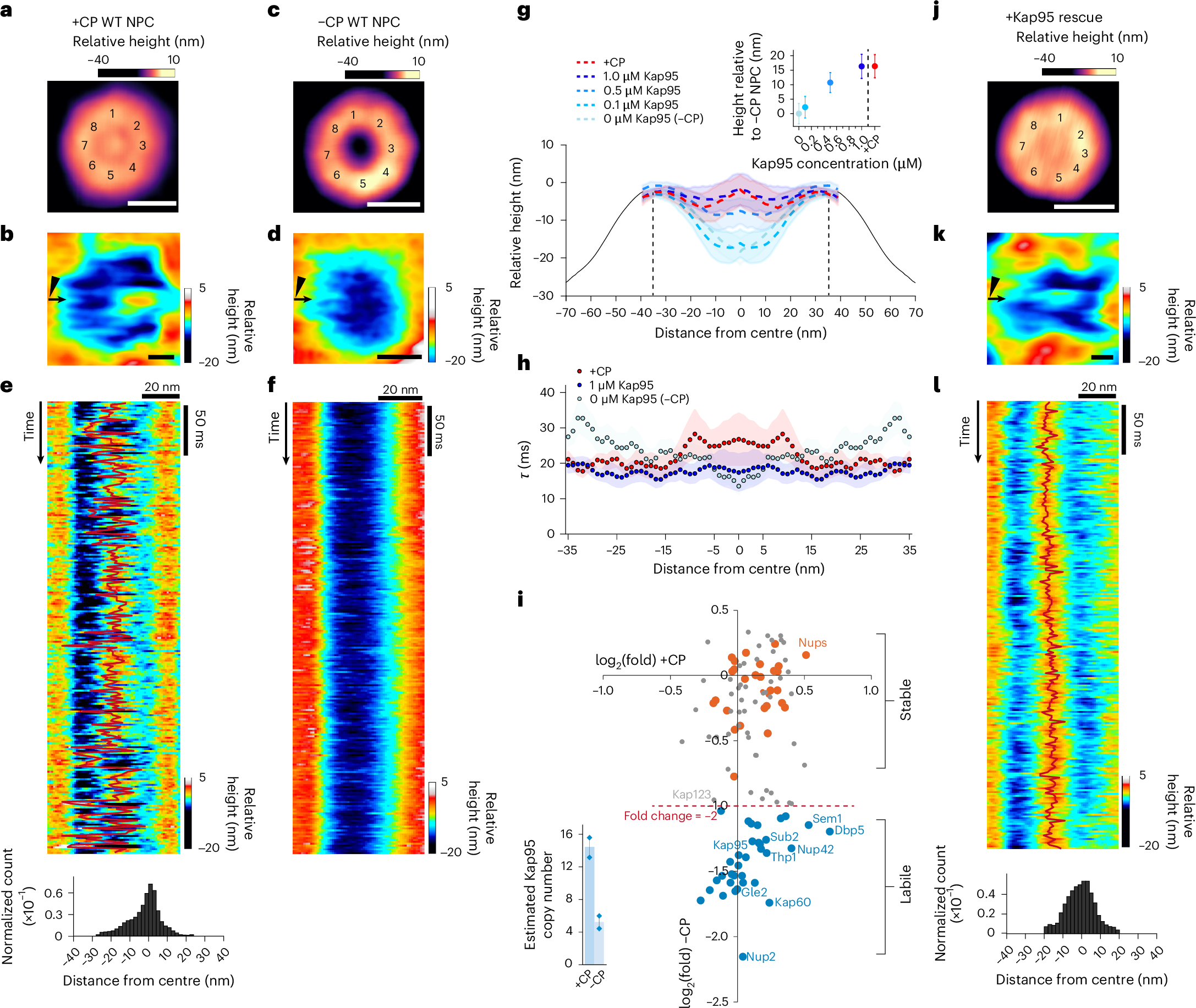
Nuclear pore complexes (NPCs) mediate selective exchange of macromolecules between the nucleus and cytoplasm, but the organization of their transport barrier has been a matter of debate. Here we used high-speed atomic force microscopy, complemented with orthogonal in vitro and in vivo approaches, to probe the dynamic behaviour of the NPC central channel at millisecond resolution. We found that nuclear transport factors dynamically remodel intrinsically disordered phenylalanine-glycine (FG) domains tethered within the NPC channel, partitioning the barrier into two zones: a rapidly fluctuating annular region and a highly mobile central plug. Increased FG-repeat density in mutant NPCs dampened barrier dynamics and impaired transport. Notably, NPC-like behaviour was recapitulated in DNA origami nanopores bearing transport factors and correctly tethered FG domains but not in in vitro FG hydrogels. Thus, the rotationally symmetric architecture of NPCs supports a nanoscopic barrier organization that contrasts with many of the bulk properties of in vitro FG-domain assemblies.