2025-12-04 インペリアル・カレッジ・ロンドン(ICL)
<関連情報>
- https://www.imperial.ac.uk/news/articles/2025/-weight-loss-drug-liraglutide-slowed-alzheimers-decline/
- https://www.nature.com/articles/s41591-025-04106-7
軽度から中等度のアルツハイマー病におけるリラグルチド:第2b相臨床試験 Liraglutide in mild to moderate Alzheimer’s disease: a phase 2b clinical trial
Paul Edison,Grazia Daniela Femminella,Craig Ritchie,Joseph Nowell,Clive Holmes,Zuzana Walker,Basil Ridha,Sanara Raza,Nicholas R. Livingston,Eleni Frangou,Sharon Love,Gareth Williams,Robert Lawrence,Brady Mcfarlane,Hilary Archer,Elizabeth Coulthard,Benjamin R. Underwood,Paul Koranteng,Salman Karim,Carol Bannister,Robert Perneczky,Aparna Prasanna,Kehinde Junaid,Bernadette McGuinness,… Clive Ballard
Nature Medicine Published:01 December 2025
DOI:https://doi.org/10.1038/s41591-025-04106-7
Abstract
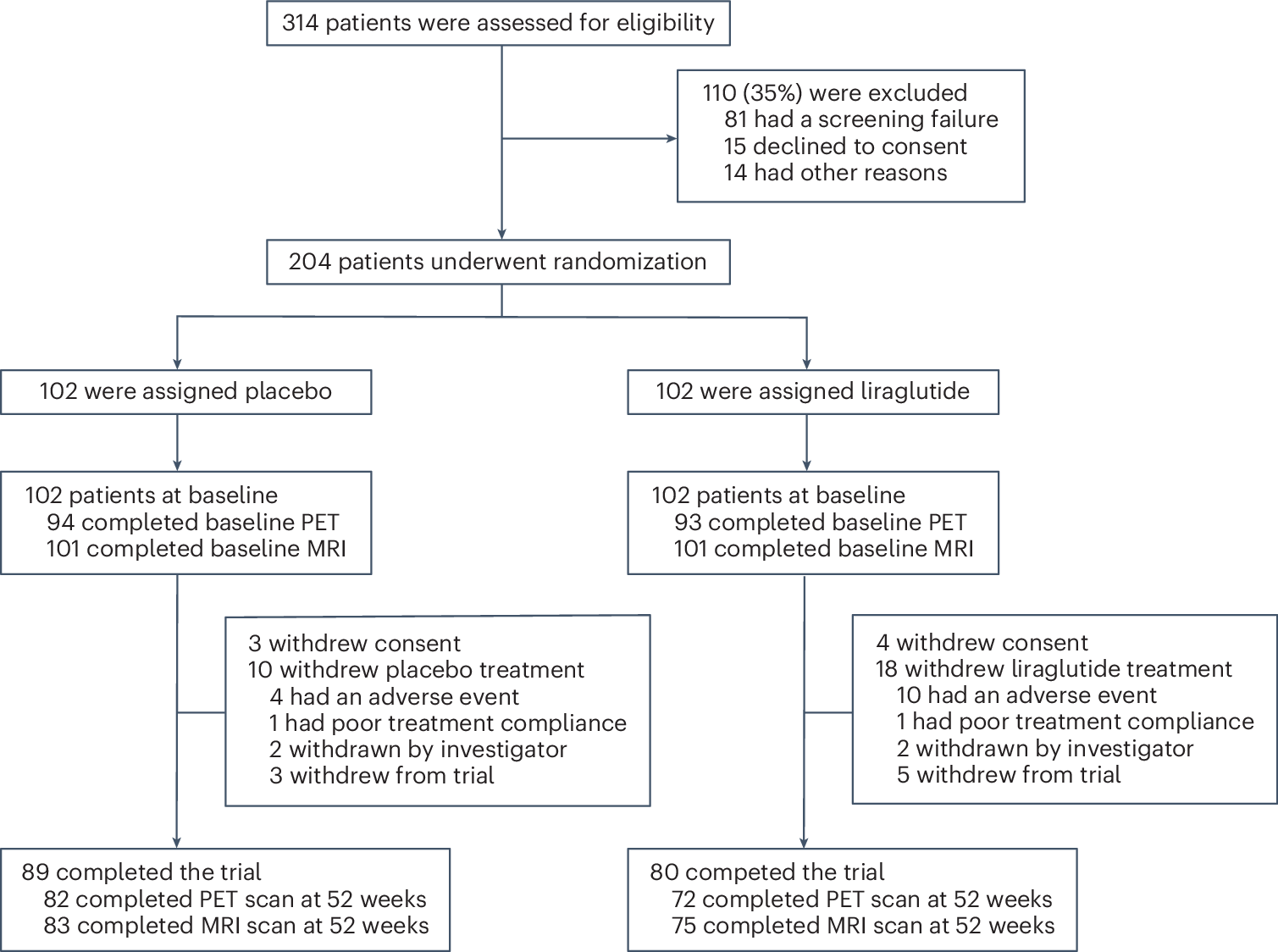
Liraglutide, a glucagon-like peptide 1 (GLP-1) agonist and antidiabetic drug, has shown neuroprotective effects in animal models. In this study, we aimed to evaluate the safety and efficacy of liraglutide in mild to moderate Alzheimer’s disease syndrome. ‘Evaluating liraglutide in Alzheimer’s disease’ (ELAD) is a multicenter, randomized, double-blind, placebo-controlled phase 2b trial in 204 participants with mild to moderate Alzheimer’s disease syndrome with no diabetes. Participants received daily injections of liraglutide or placebo for 52 weeks. They underwent fluorodeoxyglucose positron emission tomography, magnetic resonance imaging and detailed neuropsychometric evaluations. The primary outcome was a change in cerebral glucose metabolic rate. Secondary outcomes were safety and tolerability and cognitive changes. The primary outcome showed no significant differences in cerebral glucose metabolism (difference = -0.17; 95% confidence interval: -0.39 to 0.06; P = 0.14) between the two groups. The secondary outcome—score on the Alzheimer’s Disease Assessment Scale-Executive domain (ADAS-Exec)—performed better in liraglutide-treated patients compared to placebo (0.15; 95% confidence interval: 0.03-0.28; unadjusted P = 0.01). No significant differences were observed in Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL) (-0.58; 95% confidence interval: -3.13 to 1.97; unadjusted P = 0.65) or Clinical Dementia Rating-Sum of Boxes (CDR-SoB) (-0.06; 95% confidence interval: -0.57 to 0.44; unadjusted P = 0.81) scores. Liraglutide was generally safe and well tolerated in non-diabetic patients with Alzheimer’s disease. ClinicalTrials.gov identifier: NCT01843075.