2025-12-17 ウィスコンシン大学マディソン校(UW–Madison)
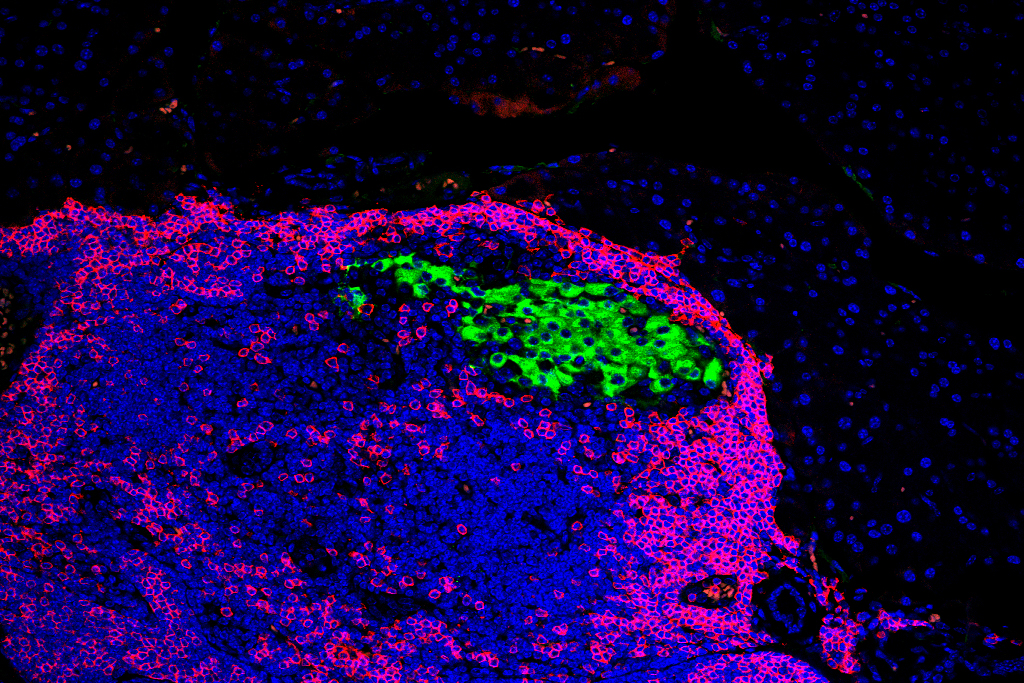
An immunofluorescence image of pancreatic cells (green) and immune cells (blue) in mice. UW researchers found that deleting a single stress-response gene in insulin-producing cells in the pancreas protects mice that are genetically predisposed to Type 1 diabetes. Courtesy of Feyza Engin
<関連情報>
- https://news.wisc.edu/how-disabling-one-gene-protects-mice-against-type-1-diabetes/
- https://www.nature.com/articles/s41467-025-65635-w
- https://www.cell.com/cell-metabolism/fulltext/S1550-4131(20)30117-0
非肥満糖尿病マウスにおけるβ細胞IRE1α/XBP1経路とその遺伝子制御ネットワーク構成要素の役割を定義する Defining the role of β-cell IRE1α/XBP1 pathway and its gene regulatory network components in non-obese diabetic mice
Hugo Lee,Khagani Eynullazada,Qiaodan Ou,Junha Shin,Sushmita Roy & Feyza Engin
Nature Communications Published:26 November 2025
DOI:https://doi.org/10.1038/s41467-025-65635-w
Abstract
The unfolded protein response sensor, IRE1α, acts through its regulated IRE1α-dependent decay (RIDD) activity or transcription factor XBP1 to determine cell fate and survival. While blunting RIDD activity prevents diabetes in type 1 diabetes preclinical model non-obese diabetic mice, β-cell-specific function of XBP1 at different stages of disease remains unknown. Here we show that deletion of Xbp1 in β-cells (Xbp1β-/-) of non-obese diabetic mice before insulitis is protective against diabetes. Histological and transcriptomic analyses indicate that following a transient loss of maturity, β-cells of Xbp1β-/- mice exhibit reduced insulitis, apoptosis, and antigenicity phenocopying Ire1αβ-/- mice with no changes in RIDD activity. Comparative transcriptome and regulatory network analyses reveal a largely shared component between the Ire1αβ-/- and Xbp1β-/- mice as well as network components unique to Xbp1β-/-, indicative of IRE1α-independent roles of XBP1. Our findings define the role of β-cell IRE1α/XBP1 and identify previously unrecognized regulatory networks and nodes of this pathway.
IRE1α欠失によって誘導されるβ細胞の脱分化は1型糖尿病を予防する Beta Cell Dedifferentiation Induced by IRE1α Deletion Prevents Type 1 Diabetes
Hugo Lee ∙ Yong-Syu Lee ∙ Quincy Harenda ∙ … ∙ Sunduz Keles ∙ Rupa Sridharan ∙ Feyza Engin
Cell Metabolism Published:March 26, 2020
DOI:https://doi.org/10.1016/j.cmet.2020.03.002
Highlights
- IRE1α deletion in NOD β cells before insulitis causes their transient dedifferentiation
- Dedifferentiated β cells show diminished expression of β cell autoantigens
- Knockout mice exhibit impaired T cell diabetogenic activity
- IRE1α-deficient NOD mice are protected from autoimmune destruction and diabetes
Summary
Immune-mediated destruction of insulin-producing β cells causes type 1 diabetes (T1D). However, how β cells participate in their own destruction during the disease process is poorly understood. Here, we report that modulating the unfolded protein response (UPR) in β cells of non-obese diabetic (NOD) mice by deleting the UPR sensor IRE1α prior to insulitis induced a transient dedifferentiation of β cells, resulting in substantially reduced islet immune cell infiltration and β cell apoptosis. Single-cell and whole-islet transcriptomics analyses of immature β cells revealed remarkably diminished expression of β cell autoantigens and MHC class I components, and upregulation of immune inhibitory markers. IRE1α-deficient mice exhibited significantly fewer cytotoxic CD8+ T cells in their pancreata, and adoptive transfer of their total T cells did not induce diabetes in Rag1-/- mice. Our results indicate that inducing β cell dedifferentiation, prior to insulitis, allows these cells to escape immune-mediated destruction and may be used as a novel preventive strategy for T1D in high-risk individuals.