2025-12-18 ロックフェラー大学
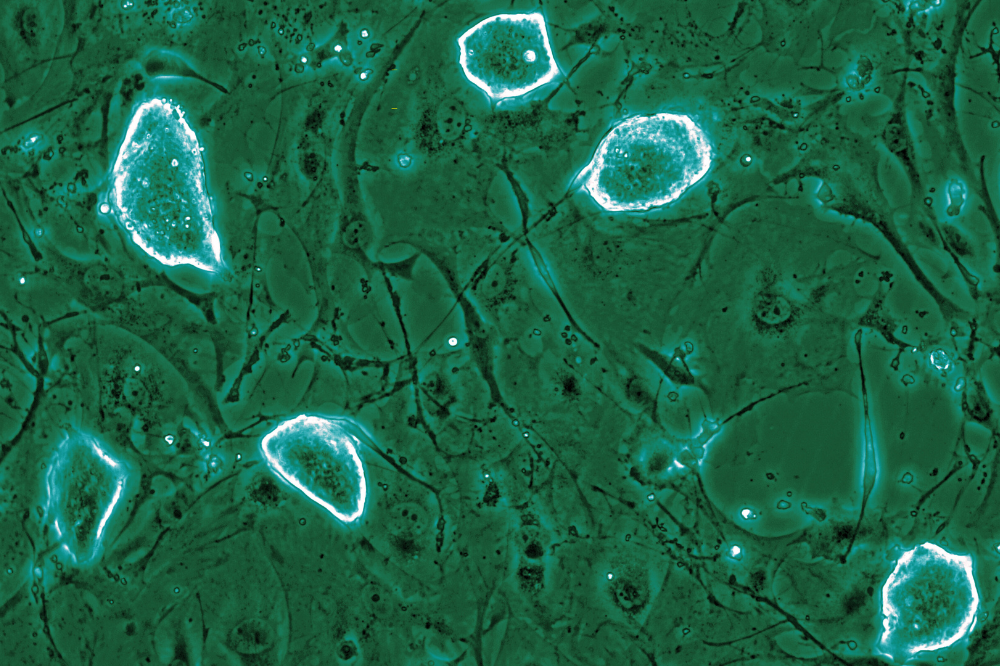
The dome shaped colonies representing diapause-like mouse embryonic stem cells. (Credit: Tarakhovsky lab)
<関連情報>
- https://www.rockefeller.edu/news/38808-some-mammals-can-hit-pause-on-a-pregnancy-understanding-how-that-happens-could-help-us-treat-cancer/
- https://pubmed.ncbi.nlm.nih.gov/41381364/
MAPキナーゼの負の制御因子の転写抑制解除は、休眠ES細胞の多能性状態の維持をサポートする Transcriptional derepression of negative regulators of MAP kinase supports maintenance of diapause ES cells in the pluripotent state
Tuo Zhang,Ryan J. Marina,Rab Prinjha,Karen Adelman and Alexander Tarakhovsky
Genes & Development Published:December 11, 2025
DOI:10.1101/gad.353143.125
Abstract
Nutrient deficiency during pregnancy in many animal species can induce embryonic diapause, a state characterized by systemic changes in biosynthetic processes that minimize reliance on external energy sources while ensuring survival. Remarkably, these changes do not affect the pluripotent state of embryonic stem (ES) cells, allowing normal development once diapause ends. Here we identify a transcriptional mechanism that maintains ES cell pluripotency during diapause. We show that inhibition of mTOR, which induces a diapause-like state in ES cells, rapidly upregulates genes encoding negative regulators of the MAP kinase (NRMAPK) pathway, a key driver of ES cell differentiation. Elevated NRMAPK expression and associated suppression of MAP kinase activity are also hallmarks of ES cells driven into diapause-like states by long-term inhibition of BET proteins, which regulate differentiation- and growth-promoting gene expression. Suppression of NRMAPK in diapause-like ES cells leads to differentiation and termination of the diapause-like state. Mechanistically, diapause-associated NRMAPK activation involves mTOR or BET inhibition-triggered release of the transcriptional repressor Capicua (CIC) from NRMAPK gene promoters. Our data highlight a key role for mTOR- and BET-controlled transcriptional regulation of MAP kinase activity via negative regulators in maintaining the pluripotent state of diapause ES cells and potentially other metabolically dormant stem or stem-like cells.