2026-01-08 中国科学院(CAS)
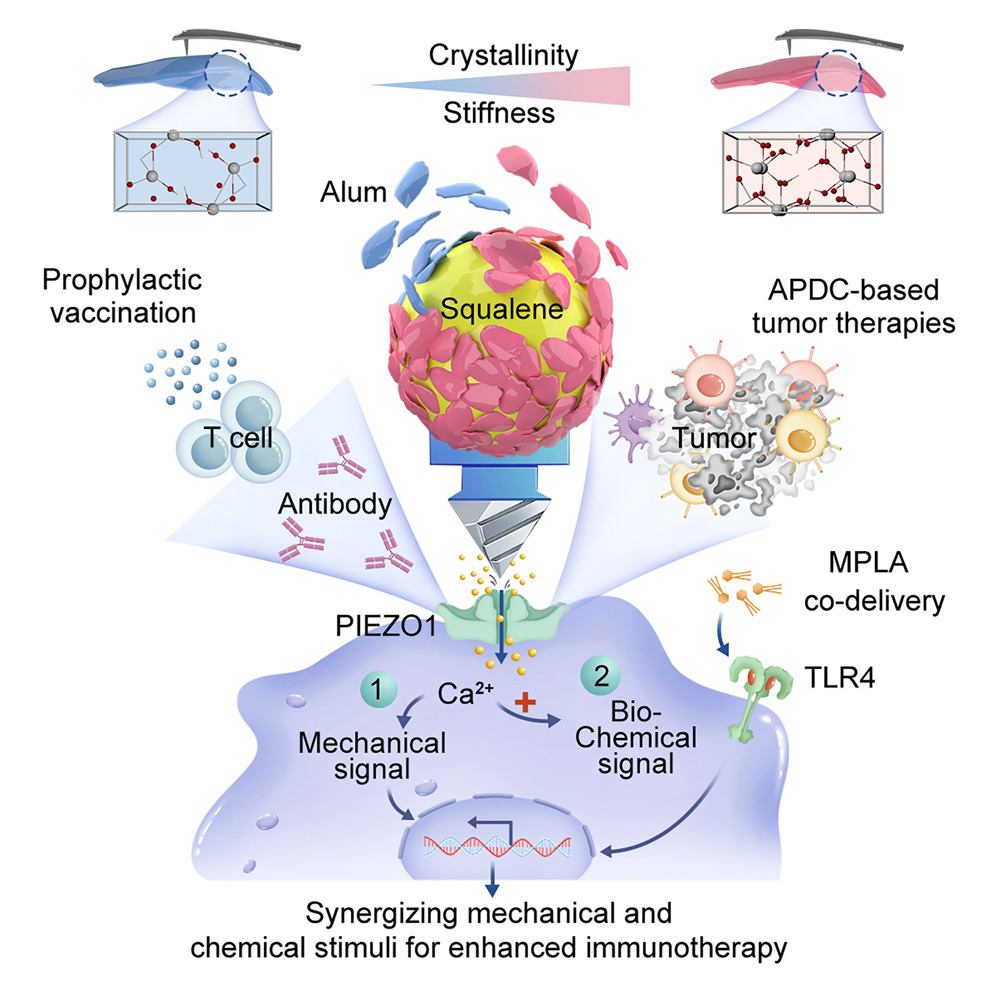
Interfacial mechano-biochemical dual signaling potentiates immune activation by Aluminum-stabilized Pickering emulsions (Image by IPE)
<関連情報>
- https://english.cas.cn/newsroom/research-news/202602/t20260210_1150308.shtml
- https://www.cell.com/cell-biomaterials/fulltext/S3050-5623(25)00272-7
樹状細胞活性化のドリリング:免疫療法強化のための界面機械的生化学的刺激の工学 Drilling dendritic cell activation: Engineering interfacial mechano-biochemical cues for enhanced immunotherapy
Yali Ming ∙ Jinji Wei ∙ Zhaoyi Zhai ∙ … ∙ Qi Huang ∙ Guanghui Ma ∙ Yufei Xia
Cell Biomaterials Published:December 10, 2025
DOI:https://doi.org/10.1016/j.celbio.2025.100281
The bigger picture
Despite progress in molecular immunoengineering, current immunotherapy strategies focus on biochemical cues, while the mechanical aspect of immune activation needs to be further explored. Here, we demonstrate that reengineering alum-based adjuvants into particle-stabilized Pickering emulsions (ASPEs) enables direct interfacial engagement with the dendritic cell membrane, triggering PIEZO1-mediated mechanotransduction. By coupling interfacial mechanics (via PIEZO1 activation) and biochemical signaling (via TLR4 stimulation), ASPEs amplify dendritic cell activation, antigen cross-presentation, and Th1-polarized immune responses. This work introduces a new framework, mechano-immunotherapy, that bridges materials science and immunology to unlock the immune potential of clinical adjuvants. Because ASPEs are based on regulatory-approved alum and simple physical restructuring, they may facilitate the clinical translation of mechanically optimized vaccines and dendritic cell therapies.
Highlights
- Reengineered alum as an interfacial mechano-adjuvant through Pickering emulsion design
- ASPEs enable direct interfacial engagement with dendritic cell membranes
- Mechanical stimulation via PIEZO1-calcium-MAPK signaling reprograms dendritic cells
- Dual activation of mechanosensing and TLR4 enhances Th1 and memory immunity
Summary
A key challenge in immunotherapy is enhancing immune responses without introducing new molecular entities that trigger regulatory hurdles. While the size, shape, and composition of approved adjuvants have been optimized, their mechanical properties remain underexplored. Here, we repurpose approved aluminum-based adjuvants (alum) by engineering alum-stabilized Pickering emulsions (ASPEs) to synergize mechanical (PIEZO1) and biochemical (TLR4) cues. ASPEs, featuring interfacial alum with optimal rigidity, were heralded to promote an enlarged contact area with dendritic cells (DCs) during endocytosis, transmitting localized stress that activates PIEZO1-mediated calcium/mitogen-activated protein kinase (MAPK) signaling. This enhances antigen cross-presentation and Th1 immunity. Co-delivering a TLR4 agonist (monophosphoryl lipid A [MPLA]) further boosted immunogenicity in a varicella-zoster virus vaccine among aged mice, outperforming alum+MPLA (AS04). In antigen-pulsed DC therapy combined with PD-1 blockade, ASPE-M-treated DCs achieved a 2.11-fold greater tumor suppression compared with tumor lysate-M-based clinical approaches. These findings demonstrate how tuning the interfacial mechanics of approved materials can unlock mechano-immunotherapy with translational potential.