2026-02-04 バッファロー大学(UB)
<関連情報>
- https://www.buffalo.edu/news/releases/2026/02/Kirkwood-anti-aging-study.html
- https://www.aginganddisease.org/EN/10.14336/AD.2025.1243
トリステトラプロリンmRNAの安定性の向上は骨の健康をサポートし、加齢に伴う骨の虚弱性を軽減する Increased Stability of Tristetraprolin mRNA Supports Bone Health and Decreases Frailty During Aging
Ramkumar Thiyagarajan, Lixia Zhang, Leticia Andrea Rojas Cortez, Kyu Hwan Kwack, Victoria Maglaras, Nanda Kumar Yellapu, Yukitomo Arao, Kenneth L. Seldeen, Perry J. Blackshear, Bruce R. Troen, Keith L. Kirkwood
Aging and Disease Published:2026-01-13
DOI:https://doi.org/10.14336/AD.2025.1243
Abstract
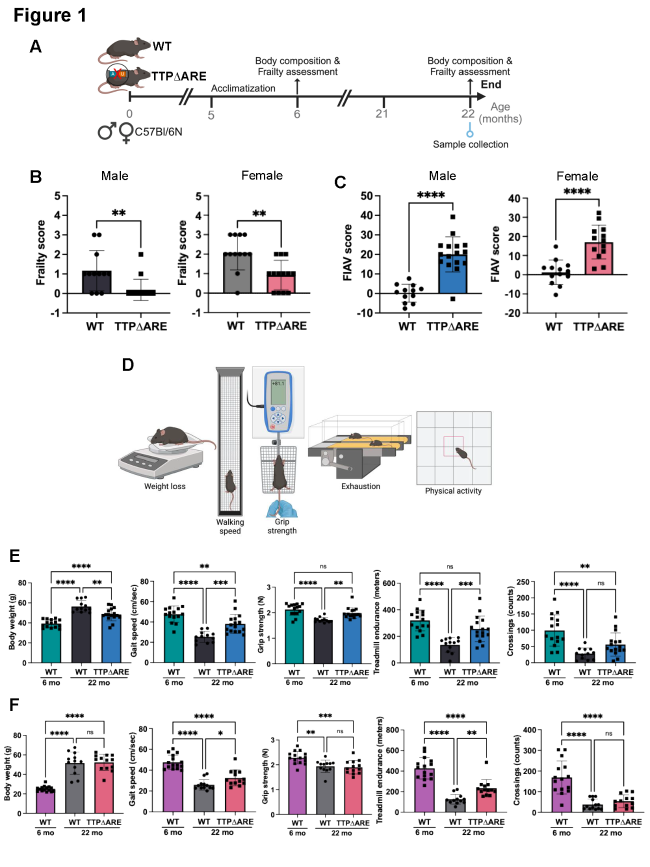
Age-related chronic low-grade inflammation contributes to both frailty and bone loss. One of the key regulators of inflammatory signaling that declines with age is tristetraprolin (TTP), an RNA-binding protein that promotes degradation of pro-inflammatory transcripts. In this study, we investigated whether stabilizing TTP during aging could reduce frailty and enhance bone health by mitigating inflammation and immune dysfunction. We utilized a knock-in mouse model (TTP∆ARE), in which an AU-rich region of the 3′ untranslated region was deleted to stabilize TTP mRNA and increase protein expression. Aged TTP∆ARE mice had reduced physical frailty scores, a composite measure based on body weight and physical performance, than age-matched wild-type controls (WT). Since frailty is associated with fracture risk, we examined bone structure. Aged TTP∆ARE males exhibited significantly higher bone mineral density and improved bone microarchitecture relative to WT mice. Our prior work showed that aging elevates myeloid-derived suppressor cells (MDSCs), which possess osteoclastogenic potential. The monocytic MDSCs (M-MDSCs) from the bone marrow of aged TTP∆ARE formed fewer osteoclasts than those from WT mice. Further, transcriptomic analysis of M-MDSCs revealed downregulation of bone resorption and remodeling pathways, along with upregulation of immune activation genes. In addition, immunophenotyping revealed a healthier, youthful-like immune profile in aged TTP∆ARE mice, including increased T-cell reservoirs. These findings signify the critical role of TTP in bone health during aging by regulating osteoimmunological induction of M-MDSCs, which leads to a partial reversal of the age-associated immune senescent phenotype, resulting in increased bone mineral density and improved functional capacity during aging.