2026-02-09 カリフォルニア大学サンディエゴ校(UCSD)
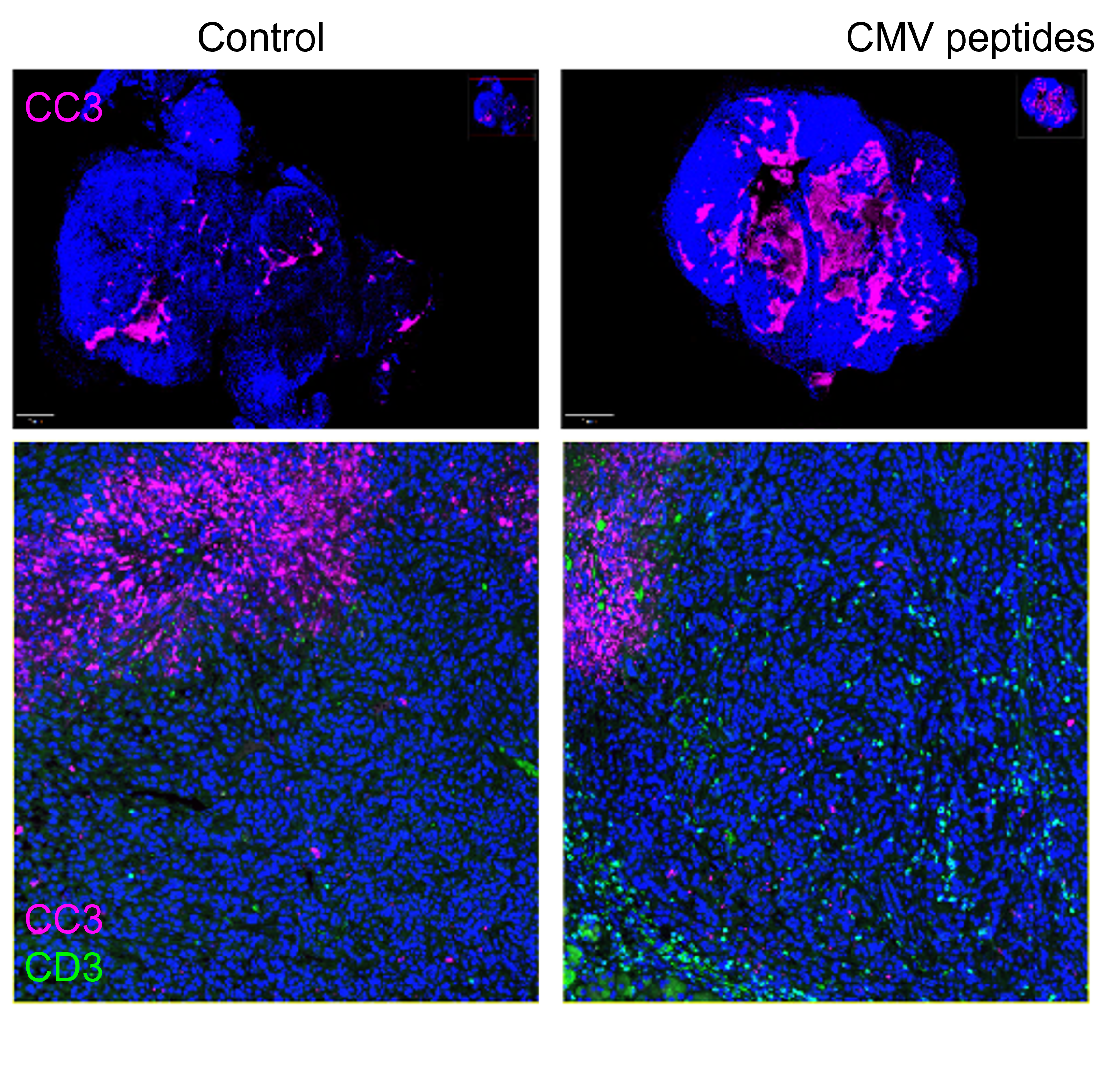 This image shows a test tumor from a CMV-infected mouse, treated with CMV peptides. Pink represents cell death. Credit: UC San Diego Health Sciences
This image shows a test tumor from a CMV-infected mouse, treated with CMV peptides. Pink represents cell death. Credit: UC San Diego Health Sciences
<関連情報>
- https://today.ucsd.edu/story/immunity-against-common-virus-leveraged-against-pancreatic-cancer
- https://jitc.bmj.com/content/14/2/e012969
膵臓癌に対するサイトメガロウイルス免疫のリダイレクトによる免疫療法 Redirecting cytomegalovirus immunity against pancreas cancer for immunotherapy
Remi Marrocco,Jay Patel,Rithika Medari,Philip Salu,Eduardo Lucero-Meza,…
Journal for ImmunoTherapy of Cancer Published:4 February 2026
Abstract
Background Immunotherapy has had limited success in pancreatic cancer, largely due to a low mutational burden and immunosuppressive microenvironment. Here we hypothesized that systemic delivery of viral antigens can redirect pre-existing antiviral immunity against pancreatic tumors.
Methods Cytomegalovirus (CMV, a β-herpesvirus) was chosen, as the majority of the population is infected and it induces an extremely large/broad memory T-cell response. Mice latently infected with murine CMV (MCMV) were orthotopically implanted with pancreatic cancer cells and treated with systemic injections of MCMV T-cell epitopes. Tumor growth was monitored by ultrasound two times a week, and immune cell infiltration was analyzed by histology, flow cytometry and single-cell RNA sequencing (scRNA-seq). Statistical analysis was performed by two-way analysis of variance with Sidak correction.
Results MCMV peptide-epitope therapy (MCMVp) promoted preferential accumulation of MCMV-specific T cells within pancreatic tumors, delaying tumor growth and increasing survival. Immunophenotyping and scRNA-seq analyses showed these T cells were highly activated and cytotoxic, leading to increased tumor necrosis and caspase-3 activation. Depletion of CD4 and CD8 T cells abolished the impact of MCMVp therapy, indicating the antitumor response is T-cell dependent. Together, these results show that CMV-specific T cells can be repurposed to combat pancreatic cancer.
Conclusions Our studies reveal that CMV-specific viral memory T cells can be re-directed to control a solid tumor normally refractory to immunotherapy via a simple, intravenous injection of T-cell peptide epitopes. This mutation-agnostic approach has significant potential for the development of “off-the-shelf” therapeutics by stimulating pre-existing antiviral memory, and it is widely applicable due to the high prevalence of CMV.


