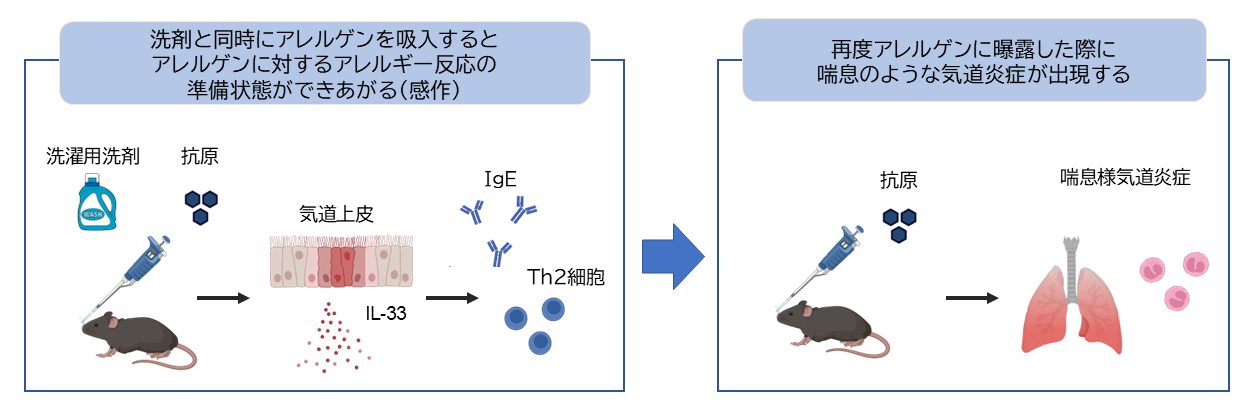
2026-02-12 北海道大学
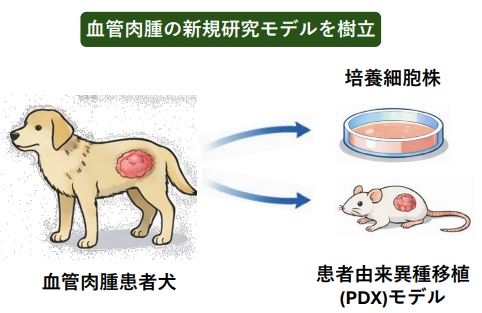
図 1. 血管肉腫研究モデルの樹立
<関連情報>
- https://www.hokudai.ac.jp/news/2026/02/post-2183.html
- https://www.hokudai.ac.jp/news/pdf/260212_pr2.pdf
- https://www.frontiersin.org/journals/veterinary-science/articles/10.3389/fvets.2026.1734339/full
イヌ血管肉腫では、グルコース欠 乏下でリジンラクチル化がATF4介在性ストレス応答を制御する
Lysine lactylation regulates ATF4-mediated stress responses under glucose starvation in canine hemangiosarcoma
Tamami Suzuki,Kazuki Heishima,Jumpei Yamazak,Masaya Yamazaki,Ryohei Kinoshita,Sangho Kim,Kenji Hosoya,Yuko Okamatsu-Ogura,Michihito Sasaki,Peng Xu;;Qin Yan,Takashi Kimura,Keisuke Aoshima
Frontiers in Veterinary Science Published:12 February 2026
DOI:https://doi.org/10.3389/fvets.2026.1734339
Excess lactate is produced in tumor cells by aerobic glycolysis and regulates gene expressions by histone lactylation. However, how histone lactylation functions under glucose-limited conditions remains unknown. Here, we show that lysine lactylation redistributes to transcription start sites (TSSs) during glucose deprivation, thereby altering biological behaviors in canine hemangiosarcoma (HSA) cells. Glucose deprivation significantly decreased global histone lactylation levels, while lactylation peaks were enriched at TSSs of ATF4-regulated stress-response, asparagine-synthesis and immune-related genes. Stress-response gene expressions were upregulated, and ATF4 polyclonal knockout abrogated this activation. [U-13C]glutamine tracing demonstrated that HSA cells synthesized asparagine from glutamine when glucose was scarce, and asparagine supplementation modestly activated cell proliferation. In HSA patient tissues, H3K18la levels were heterogeneous, and M2-like macrophages preferentially infiltrated tumor regions showing low histone lactylation levels. These findings demonstrate that lysine lactylation regulates transcription that supports tumor cell survival and fosters a pro-tumor microenvironment even under glucose-limited conditions.

.jpg)