2022-05-04 ノースカロライナ州立大学(NCState)
<関連情報>
- https://news.ncsu.edu/2022/05/faster-way-to-produce-hindered-amines/
- https://www.nature.com/articles/s41467-022-30175-0
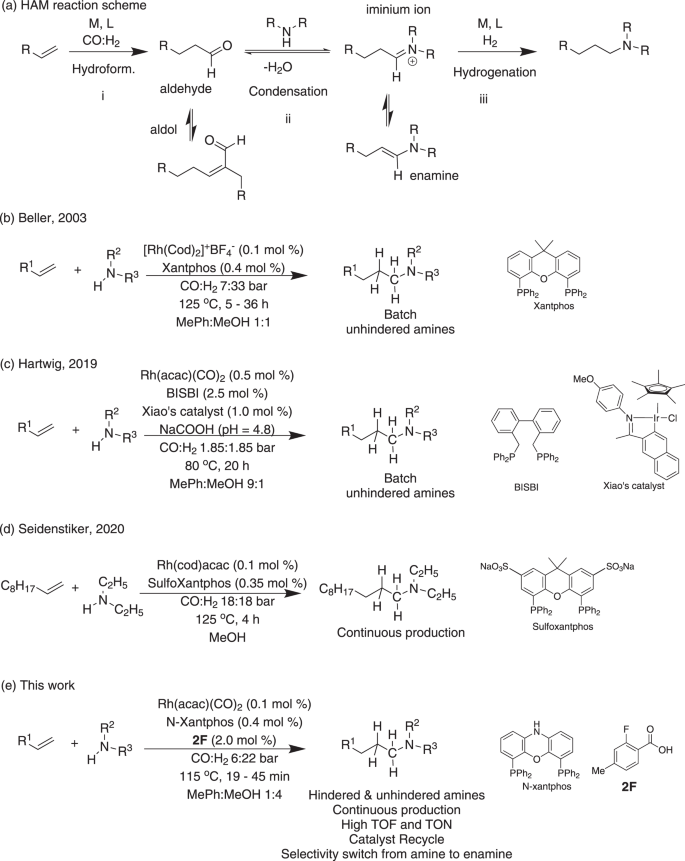
ヒンダードアミン類のヒドロアミノメチル化反応を促進するリサイクル型協同触媒の連続分割フロー反応器への適用 Recyclable cooperative catalyst for accelerated hydroaminomethylation of hindered amines in a continuous segmented flow reactor
Malek Y. S. Ibrahim & Milad Abolhasani
Nature Communications Published: 04 May 2022
DOI:https://doi.org/10.1038/s41467-022-30175-0
Abstract
Synthesis of hindered amines using the atom-efficient hydroaminomethylation (HAM) route remains a challenge. Here, we report a general and accelerated HAM in segmented flow, achieved via a cooperative effect between rhodium (Rh)/N-Xantphos and a co-catalyst (2-Fluoro-4-methylbenzoic acid) to increase the reactivity by 70 fold when compared to Rh/Xantphos in batch reactors. The cooperation between Rh and the co-catalyst facilitates the cleavage of the H–H bond and drives the equilibrium-limited condensation step forward. Online reaction optimization expands the scope to include alkyl, aryl, and primary amines. In-flow solvent tuning enables selectivity switching from amine to enamine without the need for changing the ligand. Furthermore, leveraging the ionic nature of the catalyst, we present a robust Rh recovery strategy up to 4 recycles without loss of activity.